Acylphloroglucinol Biosynthesis in Strawberry Fruit
- PMID: 26169681
- PMCID: PMC4634061
- DOI: 10.1104/pp.15.00794
Acylphloroglucinol Biosynthesis in Strawberry Fruit
Abstract
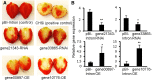
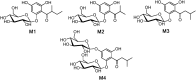
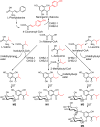
Phenolics have health-promoting properties and are a major group of metabolites in fruit crops. Through reverse genetic analysis of the functions of four ripening-related genes in the octoploid strawberry (Fragaria × ananassa), we discovered four acylphloroglucinol (APG)-glucosides as native Fragaria spp. fruit metabolites whose levels were differently regulated in the transgenic fruits. The biosynthesis of the APG aglycones was investigated by examination of the enzymatic properties of three recombinant Fragaria vesca chalcone synthase (FvCHS) proteins. CHS is involved in anthocyanin biosynthesis during ripening. The F. vesca enzymes readily catalyzed the condensation of two intermediates in branched-chain amino acid metabolism, isovaleryl-Coenzyme A (CoA) and isobutyryl-CoA, with three molecules of malonyl-CoA to form phlorisovalerophenone and phlorisobutyrophenone, respectively, and formed naringenin chalcone when 4-coumaroyl-CoA was used as starter molecule. Isovaleryl-CoA was the preferred starter substrate of FvCHS2-1. Suppression of CHS activity in both transient and stable CHS-silenced fruit resulted in a substantial decrease of APG glucosides and anthocyanins and enhanced levels of volatiles derived from branched-chain amino acids. The proposed APG pathway was confirmed by feeding isotopically labeled amino acids. Thus, Fragaria spp. plants have the capacity to synthesize pharmaceutically important APGs using dual functional CHS/(phloriso)valerophenone synthases that are expressed during fruit ripening. Duplication and adaptive evolution of CHS is the most probable scenario and might be generally applicable to other plants. The results highlight that important promiscuous gene function may be missed when annotation relies solely on in silico analysis.
© 2015 American Society of Plant Biologists. All Rights Reserved.
Figures




References
-
- Abe I, Morita H (2010) Structure and function of the chalcone synthase superfamily of plant type III polyketide synthases. Nat Prod Rep 27: 809–838 - PubMed
-
- Akiyama T, Shibuya M, Liu HM, Ebizuka Y (1999) p-Coumaroyltriacetic acid synthase, a new homologue of chalcone synthase, from Hydrangea macrophylla var. thunbergii. Eur J Biochem 263: 834–839 - PubMed
-
- Allan AC, Hellens RP, Laing WA (2008) MYB transcription factors that colour our fruit. Trends Plant Sci 13: 99–102 - PubMed
-
- Almeida JRM, D’Amico E, Preuss A, Carbone F, de Vos CHR, Deiml B, Mourgues F, Perrotta G, Fischer TC, Bovy AG, et al. (2007) Characterization of major enzymes and genes involved in flavonoid and proanthocyanidin biosynthesis during fruit development in strawberry (Fragaria × ananassa). Arch Biochem Biophys 465: 61–71 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

