Tissue Factor Residues That Modulate Magnesium-Dependent Rate Enhancements of the Tissue Factor/Factor VIIa Complex
- PMID: 26169722
- PMCID: PMC4862878
- DOI: 10.1021/acs.biochem.5b00608
Tissue Factor Residues That Modulate Magnesium-Dependent Rate Enhancements of the Tissue Factor/Factor VIIa Complex
Abstract
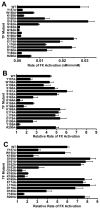
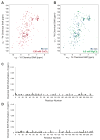
The blood coagulation cascade is initiated when the cell-surface complex of factor VIIa (FVIIa, a trypsin-like serine protease) and tissue factor (TF, an integral membrane protein) proteolytically activates factor X (FX). Both FVIIa and FX bind to membranes via their γ-carboxyglutamate-rich domains (GLA domains). GLA domains contain seven to nine bound Ca(2+) ions that are critical for their folding and function, and most biochemical studies of blood clotting have employed supraphysiologic Ca(2+) concentrations to ensure saturation of these domains with bound Ca(2+). Recently, it has become clear that, at plasma concentrations of metal ions, Mg(2+) actually occupies two or three of the divalent metal ion-binding sites in GLA domains, and that these bound Mg(2+) ions are required for full function of these clotting proteins. In this study, we investigated how Mg(2+) influences FVIIa enzymatic activity. We found that the presence of TF was required for Mg(2+) to enhance the rate of FX activation by FVIIa, and we used alanine-scanning mutagenesis to identify TF residues important for mediating this response to Mg(2+). Several TF mutations, including those at residues G164, K166, and Y185, blunted the ability of Mg(2+) to enhance the activity of the TF/FVIIa complex. Our results suggest that these TF residues interact with the GLA domain of FX in a Mg(2+)-dependent manner (although effects of Mg(2+) on the FVIIa GLA domain cannot be ruled out). Notably, these TF residues are located within or immediately adjacent to the putative substrate-binding exosite of TF.
Figures

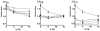

References
-
- Bloom JW, Mann KG. Metal ion induced conformational transitions of prothrombin and prothrombin fragment 1. Biochemistry. 1978;17:4430–4438. - PubMed
-
- Zwaal RFA, Comfurius P, Bevers EM. Lipid-protein interactions in blood coagulation. Biochim Biophys Acta. 1998;1376:433–453. - PubMed
-
- Sunnerhagen M, Forsén S, Hoffrén AM, Drakenberg T, Teleman O, Stenflo J. Structure of the Ca2+-free GLA domain sheds light on membrane binding of blood coagulation proteins. Nat Struct Biol. 1995;2:504–509. - PubMed
-
- Falls LA, Furie BC, Jacobs M, Furie B, Rigby AC. The ω-loop region of the human prothrombin γ-carboxyglutamic acid domain penetrates anionic phospholipid membranes. J Biol Chem. 2001;276:23895–23902. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

