Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): study protocol for a randomized controlled trial
- PMID: 26169781
- PMCID: PMC4499946
- DOI: 10.1186/s13063-015-0825-8
Maternal vitamin D supplementation during pregnancy and lactation to promote infant growth in Dhaka, Bangladesh (MDIG trial): study protocol for a randomized controlled trial
Abstract
Background: Vitamin D regulates bone mineral metabolism and skeletal development. Some observational studies have suggested that prenatal vitamin D deficiency increases the risk of adverse pregnancy and/or birth outcomes; however, there is scant evidence from controlled trials, leading the World Health Organization to advise against routine vitamin D supplementation in pregnancy. Importantly, little is known about the effect of maternal vitamin D status on infant linear growth in communities in South Asia where stunting is highly prevalent and maternal-infant vitamin D status is commonly suboptimal.
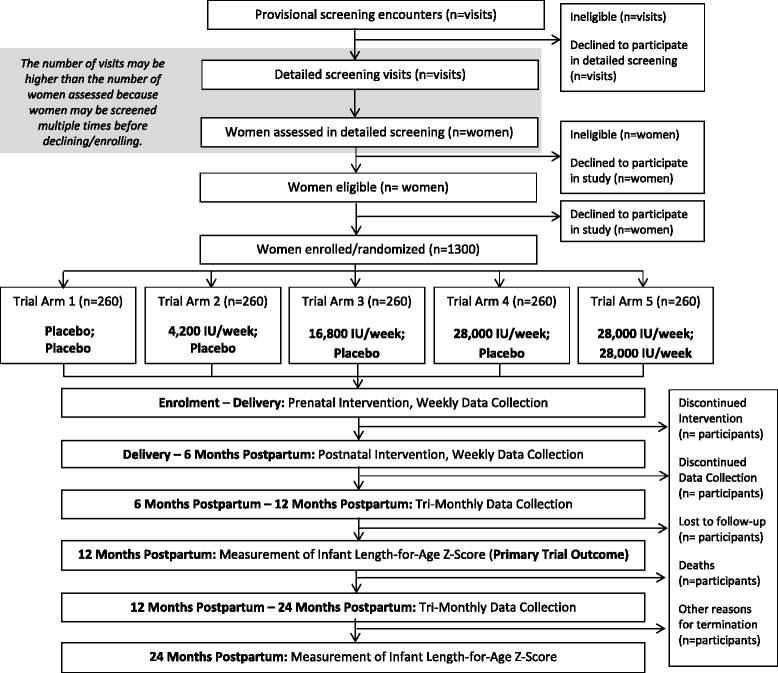
Methods/design: The Maternal Vitamin D for Infant Growth study is a randomized, placebo-controlled, dose-ranging trial of maternal vitamin D supplementation during pregnancy and lactation in Dhaka, Bangladesh. The primary aims are to estimate (1) the effect of maternal prenatal oral vitamin D3 supplementation (4200 IU/wk, 16,800 IU/wk, or 28,000 IU/wk, administered as weekly doses) versus placebo on infant length at 1 year of age and (2) the effect of maternal postpartum oral vitamin D3 supplementation (28,000 IU/wk) versus placebo on length at 1 year of age among infants born to women who received vitamin D 28,000 IU/wk during pregnancy. Generally healthy pregnant women (n = 1300) in the second trimester (17-24 weeks of gestation) are randomized to one of five parallel arms: placebo 4200 IU/wk, 16,800 IU/wk, or 28,000 IU/wk in the prenatal period and placebo in the postpartum period or 28,000 IU/wk in the prenatal period and 28,000 IU/wk in the postpartum period. Household- and clinic-based follow-up of mother-infant pairs is conducted weekly by trained personnel until 26 weeks postpartum and every 3 months thereafter. The primary trial outcome measure is length for age z-score at 1 year of age. Anthropometric measurements, clinical information, and biological specimens collected at scheduled intervals will enable the assessment of a range of maternal, perinatal, and infant outcomes.
Discussion: The role of vitamin D in maternal and infant health remains unresolved. This trial is expected to contribute unique insights into the effects of improving maternal-infant vitamin D status in a low-income setting where stunting and adverse perinatal outcomes represent significant public health burdens.
Trial registration: ClinicalTrials.gov identifier: NCT01924013. Registered on 13 August 2013.
Figures
References
-
- Stevens GA, Finucane MM, Paciorek CJ, Flaxman SR, White RA, Donner AJ, et al. Trends in mild, moderate, and severe stunting and underweight, and progress towards MDG 1 in 141 developing countries: a systematic analysis of population representative data. Lancet. 2012;380(9844):824–34. doi: 10.1016/S0140-6736(12)60647-3. - DOI - PMC - PubMed
-
- National Institute of Population Research and Training (NIPORT), Mitra and Associates, ICF International. Bangladesh demographic and health survey 2011. Dhaka, Bangladesh/Calverton, MD: NIPORT, Mitra and Associates, ICF International; January 2013. http://dhsprogram.com/pubs/pdf/FR265/FR265.pdf. Accessed 5 July 2015.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical