Effects of Temperature and Humidity on Laser Diffraction Measurements to Jet Nebulizer and Comparison with NGI
- PMID: 26169901
- PMCID: PMC4984894
- DOI: 10.1208/s12249-015-0346-5
Effects of Temperature and Humidity on Laser Diffraction Measurements to Jet Nebulizer and Comparison with NGI
Abstract
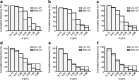
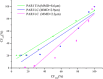
Laser diffraction (LD) and next generation impactor (NGI) are commonly used for the evaluation of inhaled drug formulations. In this study, the effect of temperature and humidity on the assessment of the nebulizer particle size distribution (PSD) by LD was investigated, and the consistency between NGI and LD measurements was evaluated. There was an increase in particle size with higher temperature or lower humidity. The particle population with a diameter less than 1 μm was significant at a temperature of 5°C or at relative humidity >90%; however, the same particle population became undetectable when temperature increased to 39°C or at relative humidity of 30-45%. The results of the NGI and LD measurements of aerosol generated from three types of jet nebulizers were compared. A poor correlation between the NGI and LD measurements was observed for PARI LC (2.2 μm) (R (2) = 0.893) and PARI LC (2.9 μm) (R (2) = 0.878), while a relatively good correlation (R (2) = 0.977) was observed for the largest particle size nebulizer (PARI TIA (8.6 μm)). We conclude that the ambient environment and the nebulizer have significant impacts on the performance and consistency between these instruments. These factors should be controlled in the evaluation of inhaled aerosol drug formulations when these instruments are used individually or in combination.
Keywords: impactor; jet nebulizer; laser diffraction; particle size distribution.
Figures



Similar articles
-
Application of a droplet evaporation model to aerodynamic size measurement of drug aerosols generated by a vibrating mesh nebulizer.J Aerosol Med Pulm Drug Deliv. 2010 Oct;23(5):295-302. doi: 10.1089/jamp.2009.0805. J Aerosol Med Pulm Drug Deliv. 2010. PMID: 20455771
-
Comparison of cascade impaction and laser diffraction for particle size distribution measurements.J Aerosol Med. 2005 Fall;18(3):311-24. doi: 10.1089/jam.2005.18.311. J Aerosol Med. 2005. PMID: 16181006
-
Determination of nebulizer droplet size distribution: a method based on impactor refrigeration.J Aerosol Med. 2007 Summer;20(2):97-104. doi: 10.1089/jam.2007.0556. J Aerosol Med. 2007. PMID: 17536948 Clinical Trial.
-
Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers.Int J Pharm. 2021 Apr 1;598:120376. doi: 10.1016/j.ijpharm.2021.120376. Epub 2021 Feb 20. Int J Pharm. 2021. PMID: 33617949
-
Design principles of liquid nebulization devices currently in use.Respir Care. 2002 Nov;47(11):1257-75; discussion 1275-8. Respir Care. 2002. PMID: 12425742 Review.
Cited by
-
Nebulised surface-active hybrid nanoparticles of voriconazole for pulmonary Aspergillosis demonstrate clathrin-mediated cellular uptake, improved antifungal efficacy and lung retention.J Nanobiotechnology. 2021 Jan 11;19(1):19. doi: 10.1186/s12951-020-00731-1. J Nanobiotechnology. 2021. PMID: 33430888 Free PMC article.
-
Characterization and comparison of Re-Du-Ning aerosol particles generated by different jet nebulizers.RSC Adv. 2019 Sep 25;9(52):30292-30301. doi: 10.1039/c9ra06177k. eCollection 2019 Sep 23. RSC Adv. 2019. PMID: 35530199 Free PMC article.
-
In vitro delivery efficiencies of nebulizers for different breathing patterns.Biomed Eng Online. 2021 Jun 10;20(1):59. doi: 10.1186/s12938-021-00895-3. Biomed Eng Online. 2021. PMID: 34112170 Free PMC article.
-
Aerosol Delivery of Dornase Alfa Generated by Jet and Mesh Nebulizers.Pharmaceutics. 2020 Jul 31;12(8):721. doi: 10.3390/pharmaceutics12080721. Pharmaceutics. 2020. PMID: 32751886 Free PMC article.
-
Comparison of Salbutamol Delivery Efficiency for Jet versus Mesh Nebulizer Using Mice.Pharmaceutics. 2019 Apr 19;11(4):192. doi: 10.3390/pharmaceutics11040192. Pharmaceutics. 2019. PMID: 31010218 Free PMC article.
References
-
- Nagao LM, Lyapustina S, Munos MK, Capizzi MD. Aspects of particle science and regulation in pharmaceutical inhalation drug products. Cryst Growth Des. 2005;5:2261–2267. doi: 10.1021/cg050224z. - DOI
-
- Le Brun PPH, De Boer AH, Heijerman HGM, Frijlink HW. A review of the technical aspects of drug nebulization. Pharm Word Sci. 2000;22:75–81. - PubMed
-
- Jaafar-Maalej C, Andrieu V, Elaissari A, Fessi H. Assessment methods of inhaled aerosols: technical aspects and applications. Drug Deliv. 2009;6:941–959. - PubMed
-
- Glenn LK, Frank PP. Metered-dose inhalers and nebulizers in the acute setting. AnnPharmacother. 1992;26:92–95. - PubMed
-
- Gibbons A, Smyth HDC. Science and technology of nebulizers and liquid-based aerosol generators. In: Hugh D.C. Smyth, Anthony J.Hickey, editors. Controlled pulmonary drug delivery. 2011. doi: 10.1007/978-1-4419-9745-6. p.232-245.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

