CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats
- PMID: 26169930
- PMCID: PMC4854624
- DOI: 10.1016/j.expneurol.2015.07.003
CD11d integrin blockade reduces the systemic inflammatory response syndrome after traumatic brain injury in rats
Abstract
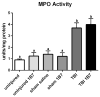
Traumatic CNS injury triggers a systemic inflammatory response syndrome (SIRS), in which circulating inflammatory cells invade body organs causing local inflammation and tissue damage. We have shown that the SIRS caused by spinal cord injury is greatly reduced by acute intravenous treatment with an antibody against the CD11d subunit of the CD11d/CD18 integrin expressed by neutrophils and monocyte/macrophages, a treatment that reduces their efflux from the circulation. Traumatic brain injury (TBI) is a frequently occurring injury after motor vehicle accidents, sporting and military injuries, and falls. Our studies have shown that the anti-CD11d treatment diminishes brain inflammation and oxidative injury after moderate or mild TBI, improving neurological outcomes. Accordingly, we examined the impact of this treatment on the SIRS triggered by TBI. The anti-CD11d treatment was given at 2h after a single moderate (2.5-3.0 atm) or 2 and 24h after each of three consecutive mild (1.0-1.5 atm) fluid percussion TBIs. Sham-injured, saline-treated rats served as controls. At 24h, 72 h, and 4 or 8 weeks after the single TBI and after the third of three TBIs, lungs of rats were examined histochemically, immunocytochemically and biochemically for downstream effects of SIRS including inflammation, tissue damage and expression of oxidative enzymes. Lung sections revealed that both the single moderate and repeated mild TBI caused alveolar disruption, thickening of inter-alveolar tissue, hemorrhage into the parenchyma and increased density of intra-and peri-alveolar macrophages. The anti-CD11d treatment decreased the intrapulmonary influx of neutrophils and the density of activated macrophages and the activity of myeloperoxidase after these TBIs. Moreover, Western blotting studies showed that the treatment decreased lung protein levels of oxidative enzymes gp91(phox), inducible nitric oxide synthase and cyclooxygenase-2, as well as the apoptotic pathway enzyme caspase-3 and levels of 4-hydroxynonenal-bound proteins (an indicator of lipid peroxidation). Decreased expression of the cytoprotective transcription factor Nrf2 reflected decreased lung oxidative stress. Anti-CD11d treatment also diminished the lung concentration of free radicals and tissue aldehydes. In conclusion, the substantial lung component of the SIRS after single or repeated TBIs is significantly decreased by a simple, minimally invasive and short-lasting anti-inflammatory treatment.
Keywords: Anti-integrin treatment; Lung; Lung damage; Systemic inflammatory response; Traumatic brain injury.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






References
-
- Acosta JA, Yang JC, Winchell RJ, Simons RK, Fortlage DA, Hollingsworth-Fridlund P, Hoyt DB. Lethal injuries and time to death in a level I trauma center. J Am Coll Surg. 1998;186:528–533. - PubMed
-
- Anthony D, Dempster R, Fearn S, Clements J, Wells G, Perry VH, Walker K. CXC chemokines generate age-related increases in neutrophil-mediated brain inflammation and blood–brain barrier breakdown. Curr Biol. 1998;8:923–926. - PubMed
-
- Baigelman W, O’Brien JC. Pulmonary effects of head trauma. Neurosurgery. 1981;9:729–740. - PubMed
-
- Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 2004;88:1335–1344. - PubMed
-
- Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 2005;94:1361–1373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

