Blocking neutrophil integrin activation prevents ischemia-reperfusion injury
- PMID: 26169939
- PMCID: PMC4516797
- DOI: 10.1084/jem.20142358
Blocking neutrophil integrin activation prevents ischemia-reperfusion injury
Abstract
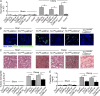
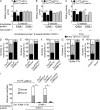
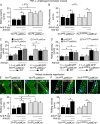
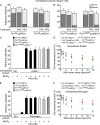
Neutrophil recruitment, mediated by β2 integrins, combats pyogenic infections but also plays a key role in ischemia-reperfusion injury and other inflammatory disorders. Talin induces allosteric rearrangements in integrins that increase affinity for ligands (activation). Talin also links integrins to actin and other proteins that enable formation of adhesions. Structural studies have identified a talin1 mutant (L325R) that perturbs activation without impairing talin's capacity to link integrins to actin and other proteins. Here, we found that mice engineered to express only talin1(L325R) in myeloid cells were protected from renal ischemia-reperfusion injury. Dissection of neutrophil function in vitro and in vivo revealed that talin1(L325R) neutrophils had markedly impaired chemokine-induced, β2 integrin-mediated arrest, spreading, and migration. Surprisingly, talin1(L325R) neutrophils exhibited normal selectin-induced, β2 integrin-mediated slow rolling, in sharp contrast to the defective slow rolling of neutrophils lacking talin1 or expressing a talin1 mutant (W359A) that blocks talin interaction with integrins. These studies reveal the importance of talin-mediated activation of integrins for renal ischemia-reperfusion injury. They further show that neutrophil arrest requires talin recruitment to and activation of integrins. However, although neutrophil slow rolling requires talin recruitment to integrins, talin-mediated integrin activation is dispensable.
© 2015 Yago et al.
Figures



Comment in
-
Renal injury: Prevention of talin-1-mediated activation of neutrophils protects against renal ischaemia-reperfusion injury.Nat Rev Nephrol. 2015 Sep;11(9):506. doi: 10.1038/nrneph.2015.130. Epub 2015 Jul 28. Nat Rev Nephrol. 2015. PMID: 26215514 No abstract available.
References
-
- Anthis N.J., Wegener K.L., Ye F., Kim C., Goult B.T., Lowe E.D., Vakonakis I., Bate N., Critchley D.R., Ginsberg M.H., and Campbell I.D.. 2009. The structure of an integrin/talin complex reveals the basis of inside-out signal transduction. EMBO J. 28:3623–3632. 10.1038/emboj.2009.287 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

