An internal pilot study for a randomized trial aimed at evaluating the effectiveness of iron interventions in children with non-anemic iron deficiency: the OptEC trial
- PMID: 26170014
- PMCID: PMC4501099
- DOI: 10.1186/s13063-015-0829-4
An internal pilot study for a randomized trial aimed at evaluating the effectiveness of iron interventions in children with non-anemic iron deficiency: the OptEC trial
Abstract
Background: The OptEC trial aims to evaluate the effectiveness of oral iron in young children with non-anemic iron deficiency (NAID). The initial sample size calculated for the OptEC trial ranged from 112-198 subjects. Given the uncertainty regarding the parameters used to calculate the sample, an internal pilot study was conducted. The objectives of this internal pilot study were to obtain reliable estimate of parameters (standard deviation and design factor) to recalculate the sample size and to assess the adherence rate and reasons for non-adherence in children enrolled in the pilot study.
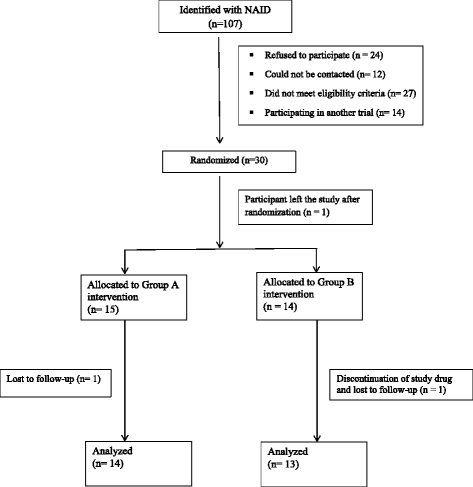
Methods: The first 30 subjects enrolled into the OptEC trial constituted the internal pilot study. The primary outcome of the OptEC trial is the Early Learning Composite (ELC). For estimation of the SD of the ELC, descriptive statistics of the 4 month follow-up ELC scores were assessed within each intervention group. The observed SD within each group was then pooled to obtain an estimated SD (S2) of the ELC. Correlation (ρ) between the ELC measured at baseline and follow-up was assessed. Recalculation of the sample size was performed using analysis of covariance (ANCOVA) method which uses the design factor (1- ρ(2)). Adherence rate was calculated using a parent reported rate of missed doses of the study intervention.
Conclusion: The new estimate of the SD of the ELC was found to be 17.40 (S2). The design factor was (1- ρ2) = 0.21. Using a significance level of 5%, power of 80%, S2 = 17.40 and effect estimate (Δ) ranging from 6-8 points, the new sample size based on ANCOVA method ranged from 32-56 subjects (16-28 per group). Adherence ranged between 14% and 100% with 44% of the children having an adherence rate ≥ 86%. Information generated from our internal pilot study was used to update the design of the full and definitive trial, including recalculation of sample size, determination of the adequacy of adherence, and application of strategies to improve adherence.
Trial registration: ClinicalTrials.gov Identifier: NCT01481766 (date of registration: November 22, 2011).
Figures
Similar articles
-
Optimizing early child development for young children with non-anemic iron deficiency in the primary care practice setting (OptEC): study protocol for a randomized controlled trial.Trials. 2015 Apr 2;16:132. doi: 10.1186/s13063-015-0635-z. Trials. 2015. PMID: 25873050 Free PMC article. Clinical Trial.
-
Randomized Trial of Oral Iron and Diet Advice versus Diet Advice Alone in Young Children with Nonanemic Iron Deficiency.J Pediatr. 2021 Jun;233:233-240.e1. doi: 10.1016/j.jpeds.2021.01.073. Epub 2021 Feb 4. J Pediatr. 2021. PMID: 33548262 Clinical Trial.
-
Home fortification of foods with multiple micronutrient powders for health and nutrition in children under two years of age (Review).Evid Based Child Health. 2013 Jan;8(1):112-201. doi: 10.1002/ebch.1895. Evid Based Child Health. 2013. PMID: 23878126 Review.
-
Effect of maternal multiple micronutrient vs iron-folic acid supplementation on infant mortality and adverse birth outcomes in rural Bangladesh: the JiVitA-3 randomized trial.JAMA. 2014 Dec 24-31;312(24):2649-58. doi: 10.1001/jama.2014.16819. JAMA. 2014. PMID: 25536256 Clinical Trial.
-
Sample size recalculation in internal pilot study designs: a review.Biom J. 2006 Aug;48(4):537-55. doi: 10.1002/bimj.200510238. Biom J. 2006. PMID: 16972704 Review.
Cited by
-
The Kusamala Program for primary caregivers of children 6-59 months of age hospitalized with severe acute malnutrition in Malawi: study protocol for a cluster-randomized controlled trial.Trials. 2017 Nov 17;18(1):550. doi: 10.1186/s13063-017-2299-3. Trials. 2017. PMID: 29149905 Free PMC article. Clinical Trial.
References
-
- Abdullah K, Thorpe KE, Mamak E, Maguire JL, Birken CS, Fehlings D, et al. Optimizing early child development for young children with non-anemic iron deficiency in the primary care practice setting (OptEC): study protocol for a randomized controlled trial. Trials. 2015;16:132. doi: 10.1186/s13063-015-0635-z. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical