Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet
- PMID: 26170063
- PMCID: PMC4862866
- DOI: 10.1016/j.bbadis.2015.07.009
Adaptive changes in amino acid metabolism permit normal longevity in mice consuming a low-carbohydrate ketogenic diet
Abstract
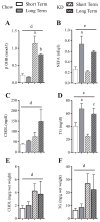
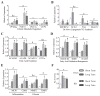
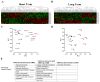
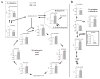
Ingestion of very low-carbohydrate ketogenic diets (KD) is associated with weight loss, lowering of glucose and insulin levels and improved systemic insulin sensitivity. However, the beneficial effects of long-term feeding have been the subject of debate. We therefore studied the effects of lifelong consumption of this diet in mice. Complete metabolic analyses were performed after 8 and 80weeks on the diet. In addition we performed a serum metabolomic analysis and examined hepatic gene expression. Lifelong consumption of KD had no effect on morbidity or mortality (KD vs. Chow, 676 vs. 630days) despite hepatic steatosis and inflammation in KD mice. The KD fed mice lost weight initially as previously reported (Kennnedy et al., 2007) and remained lighter and had less fat mass; KD consuming mice had higher levels of energy expenditure, improved glucose homeostasis and higher circulating levels of β-hydroxybutyrate and triglycerides than chow-fed controls. Hepatic expression of the critical metabolic regulators including fibroblast growth factor 21 were also higher in KD-fed mice while expression levels of lipogenic enzymes such as stearoyl-CoA desaturase-1 was reduced. Metabolomic analysis revealed compensatory changes in amino acid metabolism, primarily involving down-regulation of catabolic processes, demonstrating that mice eating KD can shift amino acid metabolism to conserve amino acid levels. Long-term KD feeding caused profound and persistent metabolic changes, the majority of which are seen as health promoting, and had no adverse effects on survival in mice.
Keywords: Free fatty acid metabolism; Hepatic steatosis; Ketogenesis; Ketogenic diet; Liver; Mass spectrometry; Metabolomics.
Copyright © 2015. Published by Elsevier B.V.
Figures


References
-
- Kennedy AR, Pissios P, Otu H, Roberson R, Xue B, Asakura K, Furukawa N, Marino FE, Liu FF, Kahn BB, Libermann TA, Maratos-Flier E. A high-fat, ketogenic diet induces a unique metabolic state in mice. Am J Physiol Endocrinol Metab. 2007;292:E1724–E1739. - PubMed
-
- Neal EG, Chaffe H, Schwartz RH, Lawson MS, Edwards N, Fitzsimmons G, Whitney A, Cross JH. The ketogenic diet for the treatment of childhood epilepsy: a randomised controlled trial. Lancet Neurol. 2008;7:500–506. - PubMed
-
- Keene DL. A systematic review of the use of the ketogenic diet in childhood epilepsy. Pediatr Neurol. 2006;35:1–5. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

