Proton block of proton-activated TRPV1 current
- PMID: 26170176
- PMCID: PMC4516785
- DOI: 10.1085/jgp.201511386
Proton block of proton-activated TRPV1 current
Abstract
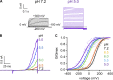
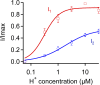
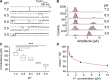
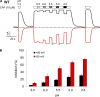
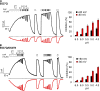
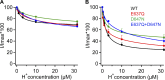
The TRPV1 cation channel is a polymodal nociceptor that is activated by heat and ligands such as capsaicin and is highly sensitive to changes in extracellular pH. In the body core, where temperature is usually stable and capsaicin is normally absent, H(+) released in response to ischemia, tissue injury, or inflammation is the best-known endogenous TRPV1 agonist, activating the channel to mediate pain and vasodilation. Paradoxically, removal of H(+) elicits a transient increase in TRPV1 current that is much larger than the initial H(+)-activated current. We found that this prominent OFF response is caused by rapid recovery from H(+) inhibition of the excitatory current carried by H(+)-activated TRPV1 channels. H(+) inhibited current by interfering with ion permeation. The degree of inhibition is voltage and permeant ion dependent, and it can be affected but not eliminated by mutations to acidic residues within or near the ion selectivity filter. The opposing H(+)-mediated gating and permeation effects produce complex current responses under different cellular conditions that are expected to greatly affect the response of nociceptive neurons and other TRPV1-expressing cells.
© 2015 Lee and Zheng.
Figures


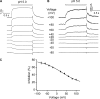
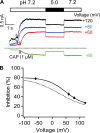

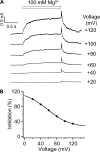
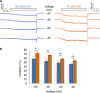


References
-
- Bers D.M. 2001. Excitation-contraction coupling and cardiac contractile force. Kluwer Academic Publishers, Norwell, MA: 427 pp10.1007/978-94-010-0658-3 - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

