Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist
- PMID: 26170295
- PMCID: PMC4522777
- DOI: 10.1073/pnas.1503617112
Cloning, synthesis, and characterization of αO-conotoxin GeXIVA, a potent α9α10 nicotinic acetylcholine receptor antagonist
Abstract
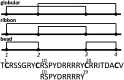
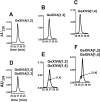
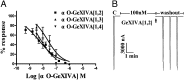
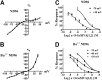
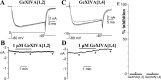
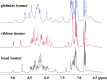
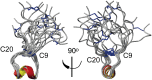
We identified a previously unidentified conotoxin gene from Conus generalis whose precursor signal sequence has high similarity to the O1-gene conotoxin superfamily. The predicted mature peptide, αO-conotoxin GeXIVA (GeXIVA), has four Cys residues, and its three disulfide isomers were synthesized. Previously pharmacologically characterized O1-superfamily peptides, exemplified by the US Food and Drug Administration-approved pain medication, ziconotide, contain six Cys residues and are calcium, sodium, or potassium channel antagonists. However, GeXIVA did not inhibit calcium channels but antagonized nicotinic AChRs (nAChRs), most potently on the α9α10 nAChR subtype (IC50 = 4.6 nM). Toxin blockade was voltage-dependent, and kinetic analysis of toxin dissociation indicated that the binding site of GeXIVA does not overlap with the binding site of the competitive antagonist α-conotoxin RgIA. Surprisingly, the most active disulfide isomer of GeXIVA is the bead isomer, comprising, according to NMR analysis, two well-resolved but uncoupled disulfide-restrained loops. The ribbon isomer is almost as potent but has a more rigid structure built around a short 310-helix. In contrast to most α-conotoxins, the globular isomer is the least potent and has a flexible, multiconformational nature. GeXIVA reduced mechanical hyperalgesia in the rat chronic constriction injury model of neuropathic pain but had no effect on motor performance, warranting its further investigation as a possible therapeutic agent.
Keywords: NMR; nicotinic; pain; α9α10 nAChR; αO-conotoxin GeXIVA.
Conflict of interest statement
Conflict of interest statement: The sequence of αO-conotoxin GeXIVA has been patented by Hainan University, with S.L., D.Z., Y.W., X.Z., Y.H., and J.M.M. listed as inventors.
Figures





References
-
- Olivera BM, Teichert RW. Diversity of the neurotoxic Conus peptides: A model for concerted pharmacological discovery. Mol Interv. 2007;7(5):251–260. - PubMed
-
- Mayer AM, et al. The odyssey of marine pharmaceuticals: A current pipeline perspective. Trends Pharmacol Sci. 2010;31(6):255–265. - PubMed
-
- Kaas Q, Westermann JC, Craik DJ. Conopeptide characterization and classifications: An analysis using ConoServer. Toxicon. 2010;55(8):1491–1509. - PubMed
-
- Gotti C, et al. Structural and functional diversity of native brain neuronal nicotinic receptors. Biochem Pharmacol. 2009;78(7):703–711. - PubMed
-
- Beech RN, Callanan MK, Rao VT, Dawe GB, Forrester SG. Characterization of cys-loop receptor genes involved in inhibitory amine neurotransmission in parasitic and free living nematodes. Parasitol Int. 2013;62(6):599–605. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

