Rsu1 regulates ethanol consumption in Drosophila and humans
- PMID: 26170296
- PMCID: PMC4522778
- DOI: 10.1073/pnas.1417222112
Rsu1 regulates ethanol consumption in Drosophila and humans
Abstract
Alcohol abuse is highly prevalent, but little is understood about the molecular causes. Here, we report that Ras suppressor 1 (Rsu1) affects ethanol consumption in flies and humans. Drosophila lacking Rsu1 show reduced sensitivity to ethanol-induced sedation. We show that Rsu1 is required in the adult nervous system for normal sensitivity and that it acts downstream of the integrin cell adhesion molecule and upstream of the Ras-related C3 botulinum toxin substrate 1 (Rac1) GTPase to regulate the actin cytoskeleton. In an ethanol preference assay, global loss of Rsu1 causes high naïve preference. In contrast, flies lacking Rsu1 only in the mushroom bodies of the brain show normal naïve preference but then fail to acquire ethanol preference like normal flies. Rsu1 is, thus, required in distinct neurons to modulate naïve and acquired ethanol preference. In humans, we find that polymorphisms in RSU1 are associated with brain activation in the ventral striatum during reward anticipation in adolescents and alcohol consumption in both adolescents and adults. Together, these data suggest a conserved role for integrin/Rsu1/Rac1/actin signaling in modulating reward-related phenotypes, including ethanol consumption, across phyla.
Keywords: actin; addiction; alcohol; genetics.
Conflict of interest statement
Conflict of interest statement: T.B. served in an advisory or consultancy role for Hexal Pharma, Lilly, Medice, Novartis, Otsuka, Oxford Outcomes, PCM Scientific, Shire, and Viforpharma. T.B. received conference attendance support and conference support or speaker’s fees from Lilly, Medice, Novartis, and Shire. T.B. is/has been involved in clinical trials conducted by Shire and Viforpharma. This work is unrelated to those grants and relationships. J.G. has received research funding from AstraZeneca, Eli Lilly & Co., Janssen-Cilag, and Bristol-Myers Squibb and speaker’s fees from AstraZeneca, Janssen-Cilag, and Bristol-Myers Squibb.
Figures
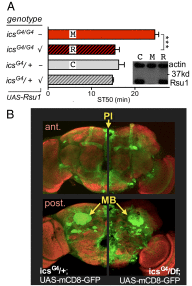
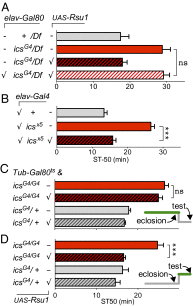
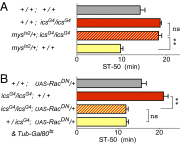

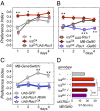
Comment in
-
Genetics of alcohol use disorders: Actin gets into the act.Proc Natl Acad Sci U S A. 2015 Nov 24;112(47):E6411. doi: 10.1073/pnas.1514716112. Epub 2015 Oct 29. Proc Natl Acad Sci U S A. 2015. PMID: 26515090 Free PMC article. No abstract available.
References
-
- World Health Organization . Global Status Report on Alcohol. World Health Organization; Geneva: 2004. pp. 65–66.
-
- Dick DM, et al. Endophenotypes successfully lead to gene identification: Results from the collaborative study on the genetics of alcoholism. Behav Genet. 2006;36(1):112–126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- F31 AA021340/AA/NIAAA NIH HHS/United States
- R01 MH063706/MH/NIMH NIH HHS/United States
- T32 DA007290/DA/NIDA NIH HHS/United States
- R01 AA019526/AA/NIAAA NIH HHS/United States
- R01MH63706:02/MH/NIMH NIH HHS/United States
- R01 GM084103/GM/NIGMS NIH HHS/United States
- 1RL1MH083268-01/MH/NIMH NIH HHS/United States
- R01AA019526/AA/NIAAA NIH HHS/United States
- K08DK091316/DK/NIDDK NIH HHS/United States
- U54 EB020403/EB/NIBIB NIH HHS/United States
- G0901858/MRC_/Medical Research Council/United Kingdom
- R01GM084103/GM/NIGMS NIH HHS/United States
- K08 DK091316/DK/NIDDK NIH HHS/United States
- G0500539/MRC_/Medical Research Council/United Kingdom
- F31AA021340/AA/NIAAA NIH HHS/United States
- G0600705/MRC_/Medical Research Council/United Kingdom
- G1002319/MRC_/Medical Research Council/United Kingdom
- DA007290/DA/NIDA NIH HHS/United States
- R01 HL087679/HL/NHLBI NIH HHS/United States
- 93558/MRC_/Medical Research Council/United Kingdom
- MC_UP_0801/1/MRC_/Medical Research Council/United Kingdom
- 5R01HL087679-02/HL/NHLBI NIH HHS/United States
- RL1 MH083268/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

