Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions
- PMID: 26170297
- PMCID: PMC4534248
- DOI: 10.1073/pnas.1510802112
Melanocortin 4 receptor mutations contribute to the adaptation of cavefish to nutrient-poor conditions
Abstract
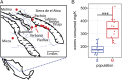
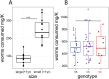
Despite recent advances in the understanding of morphological evolution, the genetic underpinnings of behavioral and physiological evolution remain largely unknown. Here, we study the metabolic changes that evolved in independently derived populations of the Mexican cavefish, Astyanax mexicanus. A hallmark of cave environments is scarcity of food. Cavefish populations rely almost entirely on sporadic food input from outside of the caves. To survive under these conditions, cavefish have evolved a range of adaptations, including starvation resistance and binge eating when food becomes available. The use of these adaptive strategies differs among independently derived cave populations. Although all cavefish populations tested lose weight more slowly than their surface conspecifics during restricted rations, only a subset of cavefish populations consume more food than their surface counterparts. A candidate gene-based screen led to the identification of coding mutations in conserved residues of the melanocortin 4 receptor (MC4R) gene, contributing to the insatiable appetite found in some populations of cavefish. Intriguingly, one of the mutated residues has been shown to be linked to obesity in humans. We demonstrate that the allele results in both reduced maximal response and reduced basal activity of the receptor in vitro. We further validate in vivo that the mutated allele contributes to elevated appetite, growth, and starvation resistance. The allele appears to be fixed in cave populations in which the overeating phenotype is present. The presence of the same allele in multiple caves appears to be due to selection from standing genetic variation present in surface populations.
Keywords: Astyanax mexicanus; MC4R; cavefish; hyperphagia; metabolic evolution.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Culver D, Pipan T. The Biology of Caves and Other Subterranean Habitats. Oxford Univ Press; Oxford: 2009.
-
- Hüppop K. Phänomene und Bedeutung der Energieersparnis bei dem Höhlenfisch Astyanax fasciatus (Characidae) Universität Hamburg; Hamburg, Germany: 1988. German.
-
- Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Dev Biol. 2001;231(1):1–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

