TACI deficiency leads to alternatively activated macrophage phenotype and susceptibility to Leishmania infection
- PMID: 26170307
- PMCID: PMC4522803
- DOI: 10.1073/pnas.1421580112
TACI deficiency leads to alternatively activated macrophage phenotype and susceptibility to Leishmania infection
Abstract
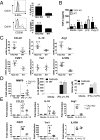
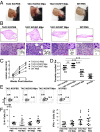
The TNF family member, transmembrane activator and calcium-modulator and cyclophilin ligand interactor (TACI), is a key molecule for plasma cell maintenance and is required in infections where protection depends on antibody response. Here, we report that compared with WT mouse, TACI KO Μϕs expressed lower levels of Toll-like receptors (TLRs), CD14, myeloid differentiation primary response protein 88, and adaptor protein Toll/IL-1 receptor domain-containing adapter-inducing IFN-β and responded poorly to TLR agonists. Analysis of Μϕ phenotype revealed that, in the absence of TACI, Μϕs adapt the alternatively activated (M2) phenotype. Steady-state expression levels for M2 markers IL-4Rα, CD206, CCL22, IL-10, Arg1, IL1RN, and FIZZ1 were significantly higher in TACI KO Μϕ than in WT cells. Confirming their M2 phenotype, TACI-KO Mϕs were unable to control Leishmania major infection in vitro, and intradermal inoculation of Leishmania resulted in a more severe manifestation of disease than in the resistant C57BL/6 strain. Transfer of WT Μϕs to TACI KO mice was sufficient to significantly reduce disease severity. TACI is likely to influence Mϕ phenotype by mediating B cell-activating factor belonging to the TNF family (BAFF) and a proliferation inducing ligand (APRIL) signals because both these ligands down-regulated M2 markers in WT but not in TACI-deficient Μϕs. Moreover, treatment of Μϕs with BAFF or APRIL enhanced the clearance of Leishmania from cells only when TACI is expressed. These findings may have implications for understanding the shortcomings of host response in newborns where TACI expression is reduced and in combined variable immunodeficiency patients where TACI signaling is ablated.
Keywords: APRIL; BAFF; Leishmania; TACI; macrophage.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
-
- Mackay F, Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. - PubMed
-
- Katsenelson N, et al. Synthetic CpG oligodeoxynucleotides augment BAFF- and APRIL-mediated immunoglobulin secretion. Eur J Immunol. 2007;37(7):1785–1795. - PubMed
-
- von Bülow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14(5):573–582. - PubMed
-
- Kanswal S, Katsenelson N, Selvapandiyan A, Bram RJ, Akkoyunlu M. Deficient TACI expression on B lymphocytes of newborn mice leads to defective Ig secretion in response to BAFF or APRIL. J Immunol. 2008;181(2):976–990. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

