High-throughput analysis of yeast replicative aging using a microfluidic system
- PMID: 26170317
- PMCID: PMC4522780
- DOI: 10.1073/pnas.1510328112
High-throughput analysis of yeast replicative aging using a microfluidic system
Abstract
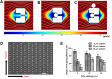
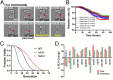
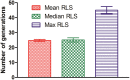
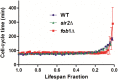
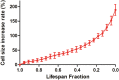
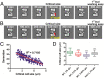
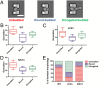
Saccharomyces cerevisiae has been an important model for studying the molecular mechanisms of aging in eukaryotic cells. However, the laborious and low-throughput methods of current yeast replicative lifespan assays limit their usefulness as a broad genetic screening platform for research on aging. We address this limitation by developing an efficient, high-throughput microfluidic single-cell analysis chip in combination with high-resolution time-lapse microscopy. This innovative design enables, to our knowledge for the first time, the determination of the yeast replicative lifespan in a high-throughput manner. Morphological and phenotypical changes during aging can also be monitored automatically with a much higher throughput than previous microfluidic designs. We demonstrate highly efficient trapping and retention of mother cells, determination of the replicative lifespan, and tracking of yeast cells throughout their entire lifespan. Using the high-resolution and large-scale data generated from the high-throughput yeast aging analysis (HYAA) chips, we investigated particular longevity-related changes in cell morphology and characteristics, including critical cell size, terminal morphology, and protein subcellular localization. In addition, because of the significantly improved retention rate of yeast mother cell, the HYAA-Chip was capable of demonstrating replicative lifespan extension by calorie restriction.
Keywords: Saccharomyces cerevisiae; calorie restriction; high-throughput; microfluidics; replicative aging.
Conflict of interest statement
The authors declare no conflict of interest.
Figures











Similar articles
-
A high-throughput microfluidic diploid yeast long-term culturing (DYLC) chip capable of bud reorientation and concerted daughter dissection for replicative lifespan determination.J Nanobiotechnology. 2022 Mar 31;20(1):171. doi: 10.1186/s12951-022-01379-9. J Nanobiotechnology. 2022. PMID: 35361237 Free PMC article.
-
Measuring the Replicative Lifespan of Saccharomyces cerevisiae Using the HYAA Microfluidic Platform.Methods Mol Biol. 2020;2144:1-6. doi: 10.1007/978-1-0716-0592-9_1. Methods Mol Biol. 2020. PMID: 32410020 Free PMC article.
-
Yeast Replicator: A High-Throughput Multiplexed Microfluidics Platform for Automated Measurements of Single-Cell Aging.Cell Rep. 2015 Oct 20;13(3):634-644. doi: 10.1016/j.celrep.2015.09.012. Epub 2015 Oct 9. Cell Rep. 2015. PMID: 26456818 Free PMC article.
-
Microfluidic technologies for yeast replicative lifespan studies.Mech Ageing Dev. 2017 Jan;161(Pt B):262-269. doi: 10.1016/j.mad.2016.03.009. Epub 2016 Mar 23. Mech Ageing Dev. 2017. PMID: 27015709 Free PMC article. Review.
-
Functional genomics of dietary restriction and longevity in yeast.Mech Ageing Dev. 2019 Apr;179:36-43. doi: 10.1016/j.mad.2019.02.003. Epub 2019 Feb 18. Mech Ageing Dev. 2019. PMID: 30790575 Review.
Cited by
-
Life span extension by glucose restriction is abrogated by methionine supplementation: Cross-talk between glucose and methionine and implication of methionine as a key regulator of life span.Sci Adv. 2020 Aug 5;6(32):eaba1306. doi: 10.1126/sciadv.aba1306. eCollection 2020 Aug. Sci Adv. 2020. PMID: 32821821 Free PMC article.
-
Multiscale effects of heating and cooling on genes and gene networks.Proc Natl Acad Sci U S A. 2018 Nov 6;115(45):E10797-E10806. doi: 10.1073/pnas.1810858115. Epub 2018 Oct 19. Proc Natl Acad Sci U S A. 2018. PMID: 30341217 Free PMC article.
-
An inexpensive microscopy system for microfluidic studies in budding yeast.Transl Med Aging. 2019;3:52-56. doi: 10.1016/j.tma.2019.05.001. Epub 2019 Jun 7. Transl Med Aging. 2019. PMID: 31511839 Free PMC article.
-
Dietary change without caloric restriction maintains a youthful profile in ageing yeast.PLoS Biol. 2023 Aug 29;21(8):e3002245. doi: 10.1371/journal.pbio.3002245. eCollection 2023 Aug. PLoS Biol. 2023. PMID: 37643155 Free PMC article.
-
Sis2 regulates yeast replicative lifespan in a dose-dependent manner.Nat Commun. 2023 Nov 27;14(1):7719. doi: 10.1038/s41467-023-43233-y. Nat Commun. 2023. PMID: 38012152 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

