Pharmacokinetics, pharmacodynamics, and safety of lesinurad, a selective uric acid reabsorption inhibitor, in healthy adult males
- PMID: 26170627
- PMCID: PMC4494180
- DOI: 10.2147/DDDT.S85193
Pharmacokinetics, pharmacodynamics, and safety of lesinurad, a selective uric acid reabsorption inhibitor, in healthy adult males
Abstract
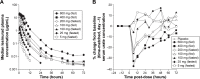
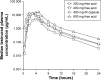
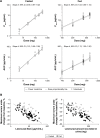
Lesinurad is a selective uric acid reabsorption inhibitor under investigation for the treatment of gout. Single and multiple ascending dose studies were conducted to evaluate pharmacokinetics, pharmacodynamics, and safety of lesinurad in healthy males. Lesinurad was administered as an oral solution between 5 mg and 600 mg (single ascending dose; N=34) and as an oral solution or immediate-release capsules once daily (qday) between 100 mg and 400 mg for 10 days under fasted or fed condition (multiple ascending dose; N=32). Following single doses of lesinurad solution, absorption was rapid and exposure (maximum observed plasma concentration and area under the plasma concentration-time curve) increased in a dose-proportional manner. Following multiple qday doses, there was no apparent accumulation of lesinurad. Urinary excretion of unchanged lesinurad was generally between 30% and 40% of dose. Increases in urinary excretion of uric acid and reductions in serum uric acid correlated with dose. Following 400 mg qday dosing, serum uric acid reduction was 35% at 24 hours post-dose, supporting qday dosing. A relative bioavailability study in healthy males (N=8) indicated a nearly identical pharmacokinetic profile following dosing of tablets or capsules. Lesinurad was generally safe and well tolerated.
Keywords: URAT1; clearance; food effect; single and multiple doses; urate lowering; urinary excretion.
Figures

References
-
- Doghramji PP, Wortmann RL. Hyperuricemia and gout: new concepts in diagnosis and management. Postgrad Med. 2012;124(6):98–109. - PubMed
-
- Gout: Novel Biologics Rev Up A Slow Market. Burlington: Decision Resources; 2010. [Accessed March 10, 2015]. Available from: http://decisionresources.com/Products-and-Services/Report?r=dbaspd1810.
-
- Zhang W, Doherty M, Pascual E, et al. EULAR Standing Committee for International Clinical Studies Including Therapeutics EULAR evidence based recommendations for gout. Part I: Diagnosis. Report of a task force of the Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT) Ann Rheum Dis. 2006;65(10):1301–1311. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

