Defining medication adherence in individual patients
- PMID: 26170639
- PMCID: PMC4494613
- DOI: 10.2147/PPA.S86249
Defining medication adherence in individual patients
Abstract
Background: The classification of patients as adherent or non-adherent to medications is typically based on an arbitrary threshold for the proportion of prescribed doses taken. Here, we define a patient as pharmacokinetically adherent if the serum drug levels resulting from his/her pattern of medication-taking behavior remained within the therapeutic range.
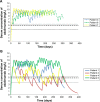
Methods: We used pharmacokinetic modeling to calculate serum drug levels in patients whose patterns of dosing were recorded by a medication event monitoring system. Medication event monitoring system data were from a previously published study of seven psoriasis patients prescribed 40 mg subcutaneous adalimumab at 14-day intervals for 1 year. Daily serum concentrations of adalimumab were calculated and compared with a known therapeutic threshold.
Results: None of the seven patients took adalimumab precisely every 14 days. Three patients who took adalimumab at intervals of 6-26 days could be classified as pharmacokinetically adherent, because their daily adalimumab serum concentration never fell below the therapeutic threshold. The four other patients, who took adalimumab at intervals of 7-93 days, could be classified as pharmacokinetically non-adherent, because their adalimumab serum concentration fell below the therapeutic threshold on 3.5%-71.3% of days.
Conclusion: Patients with varying patterns of adalimumab dosing could be classified as pharmacokinetically adherent or non-adherent according to whether or not their serum drug concentrations remained within the therapeutic range.
Keywords: adalimumab; drug administration schedule; drug therapy/utilization; patient compliance; pharmacokinetic adherence; pharmacokinetics.
Figures
References
-
- Steiner JF, Prochazka AV. The assessment of refill compliance using pharmacy records: methods, validity, and applications. J Clin Epidemiol. 1997;50(1):105–116. - PubMed
-
- Haynes RB. A critical review of the “determinants” of patient compliance with therapeutic regimens. In: Sackett DL, Haynes RB, editors. Compliance with therapeutic regimens. Baltimore, MD: Johns Hopkins University Press; 1976. pp. 26–39.
-
- Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296–1310. - PubMed
-
- Cramer JA, Roy A, Burrell A, et al. Medication compliance and persistence: terminology and definitions. Value Health. 2008;11(1):44–47. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

