Short-term use of antiepileptic drugs is neurotoxic to the immature brain
- PMID: 26170821
- PMCID: PMC4424753
- DOI: 10.4103/1673-5374.155434
Short-term use of antiepileptic drugs is neurotoxic to the immature brain
Abstract
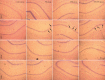
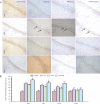
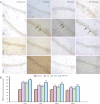
Previous studies have shown that the long-term use of antiepileptic drugs can cause nervous system damage. However, short-term antiepileptic drug treatment is frequently given to infants, especially neonates, to control seizure. Whether the short-term use of antiepileptic drugs is neurotoxic remains unclear. In the present study, immature rats, 3-21 days of age, were intraperitoneally injected with phenobarbital and/or topiramate for 3 consecutive days. Hematoxylin-eosin and immunohistochemical staining revealed that phenobarbital and topiramate, individually or in combination, were cytotoxic to hippocampal CA1 neurons and inhibited the expression of GluR1 and NR2B, excitatory glutamate receptor subunits. Furthermore, the combination of the two drugs caused greater damage than either drug alone. The results demonstrate that the short-term use of antiepileptic drugs damages neurons in the immature brain and that the combined use of antiepileptic drugs exacerbates damage. Our findings suggest that clinicians should consider the potential neurotoxic risk associated with the combined use of antiepileptic drugs in the treatment of seizure.
Keywords: NSFC grant; antiepileptic drugs; glutamate receptor; hippocampus; immature brain; nerve regeneration; neural regeneration; seizure; synaptic plasticity.
Conflict of interest statement
Figures
Similar articles
-
Effect of arachidonyl-2'-chloroethylamide, a selective cannabinoid CB1 receptor agonist, on the protective action of the various antiepileptic drugs in the mouse maximal electroshock-induced seizure model.Prog Neuropsychopharmacol Biol Psychiatry. 2010 Feb 1;34(1):18-25. doi: 10.1016/j.pnpbp.2009.09.005. Epub 2009 Sep 13. Prog Neuropsychopharmacol Biol Psychiatry. 2010. PMID: 19751793
-
[Experimental study on the possibility of brain damage induced by chronic treatment with phenobarbital, clonazepam, valproic acid and topiramate in immature rats].Zhonghua Er Ke Za Zhi. 2007 Feb;45(2):121-5. Zhonghua Er Ke Za Zhi. 2007. PMID: 17456340 Chinese.
-
Therapeutic doses of topiramate are not toxic to the developing rat brain.Exp Neurol. 2004 Jun;187(2):403-9. doi: 10.1016/j.expneurol.2004.01.025. Exp Neurol. 2004. PMID: 15144866
-
Use of antiepileptic drugs in the treatment of epilepsy in people with intellectual disability.J Intellect Disabil Res. 1998 Dec;42 Suppl 1:1-15. J Intellect Disabil Res. 1998. PMID: 10030426 Review.
-
Mechanisms of action of antiepileptic drugs.Curr Top Med Chem. 2005;5(1):3-14. doi: 10.2174/1568026053386962. Curr Top Med Chem. 2005. PMID: 15638774 Review.
Cited by
-
Neonatal Seizures in Low- and Middle-Income Countries: A Review of the Literature and Recommendations for the Management.Turk Arch Pediatr. 2024 Jan;59(1):13-22. doi: 10.5152/TurkArchPediatr.2024.23250. Turk Arch Pediatr. 2024. PMID: 38454256 Free PMC article.
-
The effects of adding prophylactic phenobarbital to therapeutic hypothermia in the term-equivalent hypoxic-ischemic rat.Pediatr Res. 2018 Feb;83(2):506-513. doi: 10.1038/pr.2017.266. Epub 2017 Nov 22. Pediatr Res. 2018. PMID: 29053702
References
-
- Auberson YP. Compititive AMPA antagonism: a novel mechanism for antiepileptic drugs? Drugs Fut. 2001;26:463–471.
-
- Aydin-Abidin S, Yildirim M, Abidin İ, Cansu A. Chronic application of topiramate and carbamazepine differentially affects the EEG and penicillin-induced epileptiform activity in rats. Neurol Res. 2012;34:246–251. - PubMed
-
- Bartha AI, Shen J, Katz KH, Mischel RE, Yap KR, Ivacko JA, Andrews EM, Ferriero DM, Ment LR, Silverstein FS. Neonatal seizures: multicenter variability in current treatment practices. Pediatr Neurol. 2007;37:85–90. - PubMed
-
- Benarroch EE. Metabotropic glutamate receptors: synaptic modulators and therapeutic targets for neurologic disease. Neurology. 2008;70:964–968. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
