The beneficial effects of adjunctive recombinant human interleukin-2 for multidrug resistant tuberculosis
- PMID: 26170852
- PMCID: PMC4495154
- DOI: 10.5114/aoms.2015.52362
The beneficial effects of adjunctive recombinant human interleukin-2 for multidrug resistant tuberculosis
Abstract
Introduction: Multidrug-resistant tuberculosis (MDR-TB) is a hard-to-treat disease with a poor outcome of chemotherapy. In the present study, the efficacy and safety of recombinant human interleukin-2 (rhIL-2) were investigated in patients with MDR-TB.
Material and methods: Fifty culture-confirmed patients with MDR-TB were included. Twenty-five patients were randomly assigned to the trial group (injection of 500 000 IU of rhIL-2 once every other day at the first, third, fifth and seventh months in addition to standard multidrug therapy) and another 25 patients to the control group with standard multidrug therapy. All patients were monitored clinically, and T-cell subsets were analyzed by flow cytometry.
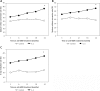
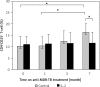
Results: The rates of sputum negative conversion and X-ray resolution in the trial group were higher than those of the control, and the improvements were significant by completion of treatment. In addition, CD4(+)CD25(+) T cells in the controls rose gradually during treatment. The levels at the end of the seventh month were significantly higher than before, which were also significantly different when compared with those from the trial group at the same time. However, there were no such changes associated with treatment in the trial group. No significant differences appeared in other T cell subsets.
Conclusions: Exogenous IL-2 in the present regimen improves immunity status. Adjunctive immunotherapy with a long period of rhIL-2 is a promising treatment modality for MDR-TB.
Keywords: CD4+CD25+ T cells; immunotherapy; interleukin-2; multidrug-resistant tuberculosis.
Figures
Similar articles
-
Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: A systematic review and meta-analysis.PLoS One. 2018 Jul 19;13(7):e0201025. doi: 10.1371/journal.pone.0201025. eCollection 2018. PLoS One. 2018. PMID: 30024982 Free PMC article.
-
Adjunctive Zoledronate + IL-2 administrations enhance anti-tuberculosis Vγ2Vδ2 T-effector populations, and improve treatment outcome of multidrug-resistant tuberculosis1.Emerg Microbes Infect. 2022 Dec;11(1):1790-1805. doi: 10.1080/22221751.2022.2095930. Emerg Microbes Infect. 2022. PMID: 35765887 Free PMC article.
-
Delamanid, linezolid, levofloxacin, and pyrazinamide for the treatment of patients with fluoroquinolone-sensitive multidrug-resistant tuberculosis (Treatment Shortening of MDR-TB Using Existing and New Drugs, MDR-END): study protocol for a phase II/III, multicenter, randomized, open-label clinical trial.Trials. 2019 Jan 16;20(1):57. doi: 10.1186/s13063-018-3053-1. Trials. 2019. PMID: 30651149 Free PMC article.
-
Decrease in CD4+CD25+FoxP3+ Treg cells after pulmonary resection in the treatment of cavity multidrug-resistant tuberculosis.Int J Infect Dis. 2010 Sep;14(9):e815-22. doi: 10.1016/j.ijid.2010.04.005. Epub 2010 Jul 22. Int J Infect Dis. 2010. PMID: 20655262
-
[Development of antituberculous drugs: current status and future prospects].Kekkaku. 2006 Dec;81(12):753-74. Kekkaku. 2006. PMID: 17240921 Review. Japanese.
Cited by
-
Clinical and Immunological Effects of rhIL-2 Therapy in Eastern Chinese Patients with Multidrug-resistant Tuberculosis.Sci Rep. 2017 Dec 19;7(1):17854. doi: 10.1038/s41598-017-18200-5. Sci Rep. 2017. PMID: 29259310 Free PMC article. Clinical Trial.
-
Understanding the Reciprocal Interplay Between Antibiotics and Host Immune System: How Can We Improve the Anti-Mycobacterial Activity of Current Drugs to Better Control Tuberculosis?Front Immunol. 2021 Jun 28;12:703060. doi: 10.3389/fimmu.2021.703060. eCollection 2021. Front Immunol. 2021. PMID: 34262571 Free PMC article. Review.
-
Recent Progress and Challenges for Drug-Resistant Tuberculosis Treatment.Pharmaceutics. 2021 Apr 21;13(5):592. doi: 10.3390/pharmaceutics13050592. Pharmaceutics. 2021. PMID: 33919204 Free PMC article. Review.
-
Therapeutic effects of recombinant human interleukin 2 as adjunctive immunotherapy against tuberculosis: A systematic review and meta-analysis.PLoS One. 2018 Jul 19;13(7):e0201025. doi: 10.1371/journal.pone.0201025. eCollection 2018. PLoS One. 2018. PMID: 30024982 Free PMC article.
-
Safety of low-dose subcutaneous recombinant interleukin-2: systematic review and meta-analysis of randomized controlled trials.Sci Rep. 2019 May 9;9(1):7145. doi: 10.1038/s41598-019-43530-x. Sci Rep. 2019. PMID: 31073219 Free PMC article.
References
-
- World Health Organization. Multidrug and extensively drug-resistant TB (M/XDR-TB): 2010 global report on surveillance and response; Geneva, Switzerland: WHO; 2010. http://www.who.int/tb/publications/2010/mdrtb2010report_executive_summar.... Accessed July 2012.m, WHO/HTM/TB/2010.3.
-
- Mercedes GJ. Immunity to TB and targets for immunotherapy. Immunotherapy. 2012;4:187–99. - PubMed
-
- Uhlin M, Andersson J, Zumla A, Maeurer M. Adjunct immunotherapies for tuberculosis. J Infect Dis. 2012;205:S325–34. - PubMed
-
- Churchyard GJ, Kaplan G, Fallows D, Wallis RS, Onyebujoh P, Rook GA. Advances in immunotherapy for tuberculosis treatment. Clin Chest Med. 2009;30:769–82. - PubMed
-
- Philips JA, Ernst JD. Tuberculosis pathogenesis and immunity. Ann Rev Pathol Mech Dis. 2012;7:353–84. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
