Hispolon inhibits TPA-induced invasion by reducing MMP-9 expression through the NF-κB signaling pathway in MDA-MB-231 human breast cancer cells
- PMID: 26171065
- PMCID: PMC4487147
- DOI: 10.3892/ol.2015.3220
Hispolon inhibits TPA-induced invasion by reducing MMP-9 expression through the NF-κB signaling pathway in MDA-MB-231 human breast cancer cells
Abstract
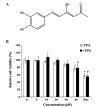
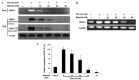
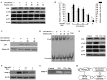
Hispolon has been demonstrated to possess analgesic, anti-inflammatory and anticancer activities. However, whether hispolon prevents the invasion of breast carcinoma cells and the underlying mechanisms of its action remain unknown. In the present study, various assays, including a matrigel-based Transwell invasion assay and electrophoretic mobility shift assay, were used to investigate the anti-invasion effect of hispolon and explore its mechanism of action. The results revealed that hispolon inhibited the 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced migration and invasion of MDA-MB-231 human breast cancer cells at non-toxic concentrations. Hispolon also prevented the TPA-induced secretion of matrix metalloproteinase-9 (MMP-9) and reduced its expression at the transcriptional and translational levels. Furthermore, the phosphorylation of IκBα was reduced by hispolon, which resulted in the suppression of nuclear factor-κB (NF-κB), and p65 phosphorylation and nuclear translocation. An electrophoretic mobility shift assay demonstrated that NF-κB DNA-binding activity was induced by TPA and inhibited by hispolon. In addition, Bay 11-7082, which is a specific inhibitor of NF-κB, functioned in a similar manner as hispolon and blocked the secretion and expression of MMP-9. In conclusion, the results of the present study indicated that hispolon inhibited TPA-induced migration and invasion of MDA-MB-231 cells by reducing the secretion and expression of MMP-9 through the NF-κB signaling pathway.
Keywords: MDA-MB-231 cancer cells; hispolon; invasion; matrix metalloproteinase-9; nuclear factor-κB.
Figures

Similar articles
-
Praeruptorin-B Inhibits 12-O-Tetradecanoylphorbol-13-Acetate-Induced Cell Invasion by Targeting AKT/NF-κB via Matrix Metalloproteinase-2/-9 Expression in Human Cervical Cancer Cells.Cell Physiol Biochem. 2019;52(6):1255-1266. doi: 10.33594/000000088. Cell Physiol Biochem. 2019. PMID: 31026389
-
Methanol extract of Codium fragile inhibits tumor necrosis factor-α-induced matrix metalloproteinase-9 and invasiveness of MDA-MB-231 cells by suppressing nuclear factor-κB activation.Asian Pac J Trop Med. 2016 Jun;9(6):535-41. doi: 10.1016/j.apjtm.2016.04.010. Epub 2016 Apr 16. Asian Pac J Trop Med. 2016. PMID: 27262063
-
Curcumol Suppresses Breast Cancer Cell Metastasis by Inhibiting MMP-9 Via JNK1/2 and Akt-Dependent NF-κB Signaling Pathways.Integr Cancer Ther. 2016 Jun;15(2):216-25. doi: 10.1177/1534735416642865. Epub 2016 Apr 28. Integr Cancer Ther. 2016. PMID: 27125675 Free PMC article.
-
12-O-tetradecanoylphorbol-13-acetate-induced invasion/migration of glioblastoma cells through activating PKCalpha/ERK/NF-kappaB-dependent MMP-9 expression.J Cell Physiol. 2010 Nov;225(2):472-81. doi: 10.1002/jcp.22226. J Cell Physiol. 2010. PMID: 20458747
-
Hispolon: A natural polyphenol and emerging cancer killer by multiple cellular signaling pathways.Environ Res. 2020 Nov;190:110017. doi: 10.1016/j.envres.2020.110017. Epub 2020 Aug 6. Environ Res. 2020. PMID: 32768475 Free PMC article. Review.
Cited by
-
Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges.Pharmaceuticals (Basel). 2023 Jan 10;16(1):101. doi: 10.3390/ph16010101. Pharmaceuticals (Basel). 2023. PMID: 36678599 Free PMC article. Review.
-
Augmentation of Docetaxel-Induced Cytotoxicity in Human PC-3 Androgen-Independent Prostate Cancer Cells by Combination With Four Natural Apoptosis-Inducing Anticancer Compounds.Nat Prod Commun. 2023 May;18(5):10.1177/1934578x231175323. doi: 10.1177/1934578x231175323. Epub 2023 May 22. Nat Prod Commun. 2023. PMID: 37292146 Free PMC article.
-
Effect of captopril on radiation-induced TGF-β1 secretion in EA.Hy926 human umbilical vein endothelial cells.Oncotarget. 2017 Mar 28;8(13):20842-20850. doi: 10.18632/oncotarget.15356. Oncotarget. 2017. PMID: 28209920 Free PMC article.
-
Novel recombinant protein FlaA N/C increases tumor radiosensitivity via NF-κB signaling in murine breast cancer cells.Oncol Lett. 2016 Oct;12(4):2632-2640. doi: 10.3892/ol.2016.4957. Epub 2016 Aug 5. Oncol Lett. 2016. PMID: 27703525 Free PMC article.
-
Attenuation of Lipopolysaccharide-Induced Acute Lung Injury by Hispolon in Mice, Through Regulating the TLR4/PI3K/Akt/mTOR and Keap1/Nrf2/HO-1 Pathways, and Suppressing Oxidative Stress-Mediated ER Stress-Induced Apoptosis and Autophagy.Nutrients. 2020 Jun 10;12(6):1742. doi: 10.3390/nu12061742. Nutrients. 2020. PMID: 32532087 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
