Vascular endothelial growth factor receptor tyrosine kinase inhibitors versus bevacizumab in metastatic colorectal cancer: A systematic review and meta-analysis
- PMID: 26171215
- PMCID: PMC4486826
- DOI: 10.3892/mco.2015.572
Vascular endothelial growth factor receptor tyrosine kinase inhibitors versus bevacizumab in metastatic colorectal cancer: A systematic review and meta-analysis
Abstract
Bevacizumab has demonstrated a survival benefit in patients with metastatic colorectal cancer (mCRC) when combined with chemotherapy. Several randomized clinical trials comparing the efficacy and toxicity of vascular endothelial growth factor receptor (VEGFR) tyrosine kinase inhibitors (TKIs) against bevacizumab have been reported. The present meta-analysis was conducted to identify the potentially significant benefit of the combined treatment regimens in patients with mCRC. PubMed, Embase and Cochrane Library databases were searched for the randomized controlled trials published on or before September 2014, which compared the efficacy and toxicity of VEGFR TKIs with bevacizumab in combination with chemotherapy in patients with mCRC. The primary endpoints included progression-free survival (PFS), overall survival (OS) and overall response rate (ORR), and secondary endpoints were the toxicity profiles. Relative risks (RRs) with 95% confidence intervals (CIs) for response rate and adverse events (AEs) were calculated, as well as hazard ratios (HRs) for PFS and OS. The final analysis included 4 studies comprising a total of 1,929 intent-to-treat patients with mCRC, which compared VEGFR TKIs (cediranib and axitinib) plus chemotherapy with bevacizumab plus chemotherapy. Results demonstrated that VEGFR TKIs plus chemotherapy significantly resulted in a modest but significantly shorter PFS [hazard ratio (HR), 1.12; 95% CI, 1.00-1.25; P=0.05] compared with that of bevacizumab plus chemotherapy but not in OS (HR, 1.10; 95% CI, 0.88-1.17; P=0.87) and ORR (RR, 0.95; 95% CI, 0.85-1.05; P=0.30). VEGFR TKIs treatment showed a less favorable AE profile compared with bevacizumab, with higher rates of grade-III/IV diarrhea, fatigue, hypertension, neutropenia and thrombocytopenia, whereas a higher incidence of peripheral neuropathy associated with the bevacizumab group was observed. In conclusion, the addition of VEGFR TKIs to chemotherapy resulted in a modest but significantly shorter PFS but not in OS and ORR compared with bevacizumab. The VEGFR TKIs group showed a less favorable AE profile with higher rates of diarrhea, fatigue, hypertension, neutropenia and thrombocytopenia, whereas a higher incidence of peripheral neuropathy associated with the bevacizumab was observed.
Keywords: advanced or metastatic colorectal cancer; bevacizumab; chemotherapy; meta-analysis; vascular endothelial growth factor receptor tyrosine kinase inhibitor.
Figures


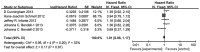



References
-
- Engstrom PF, Arnoletti JP, Benson AB, III, Chen YJ, Choti MA, Cooper HS, Covey A, Dilawari RA, Early DS, Enzinger PC, et al. National Comprehensive Cancer Network: NCCN Clinical Practice Guidelines in Oncology: Colon cancer. J Natl Compr Canc Netw. 2009;7:778–831. - PubMed
-
- Tournigand C, André T, Achille E, Lledo G, Flesh M, Mery-Mignard D, Quinaux E, Couteau C, Buyse M, Ganem G, et al. FOLFIRI followed by FOLFOX6 or the reverse sequence in advanced colorectal cancer: A randomized GERCOR study. J Clin Oncol. 2004;22:229–237. doi: 10.1200/JCO.2004.05.113. - DOI - PubMed
-
- Giantonio BJ, Catalano PJ, Meropol NJ, O'Dwyer PJ, Mitchell EP, Alberts SR, Schwartz MA, Benson AB., III Eastern Cooperative Oncology Group Study E3200: Bevacizumab in combination with oxaliplatin, fluorouracil, and leucovorin (FOLFOX4) for previously treated metastatic colorectal cancer: Results from the Eastern Cooperative Oncology Group Study E3200. J Clin Oncol. 2007;25:1539–1544. doi: 10.1200/JCO.2006.09.6305. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
