Pharmacokinetic study of metopimazine by oral route in children
- PMID: 26171218
- PMCID: PMC4492748
- DOI: 10.1002/prp2.130
Pharmacokinetic study of metopimazine by oral route in children
Abstract
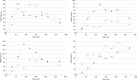
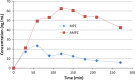
Metopimazine (MPZ) is an antiemetic considered as a currently used drug. In France, it has become the leading antiemetic mediator due to its good tolerance, however, its pharmacokinetics has never previously been studied in children. MPZ was administered by oral route to 8 children with a single dose of 0.33 mg/kg during an endocrine exploration using stimuli well known for its adverse emetic effects. We used biological remnants from sera following an hGH test in order to obtain the MPZ pharmacokinetics. Plasmatic concentrations of MPZ and the active acid metabolite AMPZ, were quantified by HPLC-MS/MS during a 270 min test period. MPZ is quickly absorbed with a median C max of 17.2 ng/mL at one hour and its half-life is 2.18 h. The plasmatic concentrations of AMPZ were higher than MPZ with a median C max of 76.3 ng/mL, a T max to 150 min and its concentration was approximately maintained at 50 ng/mL from 1 to 4 h. The plasmatic concentrations in children are similar to those observed in adults. No adverse effects, nausea or vomiting occurred during the trial. Therefore, these results confirm the MPZ dosage that should be used in children under 15 kg administered as 0.33 mg/kg up to 3 times a day.
Keywords: Antiemetic drug; children; metopimazine; pharmacokinetics.
Figures
References
-
- ANSM. 2011. Médicaments à base de dompéridone et sécurité d’emploi cardiovasculaire - Lettre aux professionnels de santé du 06/12/2011.
-
- ANSM. 2012. Contre-indication des spécialités à base de métoclopramide (Primpéran® et génériques) chez l’enfant et l’adolescent et renforcement des informations sur les risques neurologiques et cardiovasculaires - Lettre aux professionnels de santé du 08/02/2012.
-
- Dillman JR, Smith EA, Khalatbari S, Strouse PJ. I.v. glucagon use in Pediatric MR enterography: effect on image quality, length of examination, and patient tolerance. AJR Am J Roentgenol. 2013;201:185–189. - PubMed
-
- Bounoure F, Lahiani SkibaM, Besnard M, Arnaud P, Mallet E, Skiba M. Effect of iontophoresis and penetration enhancers on transdermal absorption of metopimazine. J Dermatol Sci. 2008;52:170–177. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources