Analysis of drug combinations: current methodological landscape
- PMID: 26171228
- PMCID: PMC4492765
- DOI: 10.1002/prp2.149
Analysis of drug combinations: current methodological landscape
Erratum in
-
Corrigendum.Pharmacol Res Perspect. 2019 Dec;7(6):e00549. doi: 10.1002/prp2.549. Pharmacol Res Perspect. 2019. PMID: 31859462 Free PMC article. No abstract available.
Abstract
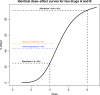
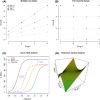
Combination therapies exploit the chances for better efficacy, decreased toxicity, and reduced development of drug resistance and owing to these advantages, have become a standard for the treatment of several diseases and continue to represent a promising approach in indications of unmet medical need. In this context, studying the effects of a combination of drugs in order to provide evidence of a significant superiority compared to the single agents is of particular interest. Research in this field has resulted in a large number of papers and revealed several issues. Here, we propose an overview of the current methodological landscape concerning the study of combination effects. First, we aim to provide the minimal set of mathematical and pharmacological concepts necessary to understand the most commonly used approaches, divided into effect-based approaches and dose-effect-based approaches, and introduced in light of their respective practical advantages and limitations. Then, we discuss six main common methodological issues that scientists have to face at each step of the development of new combination therapies. In particular, in the absence of a reference methodology suitable for all biomedical situations, the analysis of drug combinations should benefit from a collective, appropriate, and rigorous application of the concepts and methods reviewed here.
Keywords: Bliss; combination index; drug combination; loewe; synergy.
Figures


References
-
- Belen’kii MS, Schinazi RF. Multiple drug effect analysis with confidence interval. Antiviral Res. 1994;25:1–11. - PubMed
-
- Berenbaum MC. A method for testing for synergy with any number of agents. J Infect Dis. 1978;137:122–130. - PubMed
-
- Berenbaum M. What is synergy? Pharmacol Rev. 1989;41:93–141. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

