Lymphocytes as an Indicator for Initial Kidney Function: A Single Center Analysis of Outcome after Alemtuzumab or Basiliximab Induction
- PMID: 26171403
- PMCID: PMC4480808
- DOI: 10.1155/2015/985460
Lymphocytes as an Indicator for Initial Kidney Function: A Single Center Analysis of Outcome after Alemtuzumab or Basiliximab Induction
Abstract
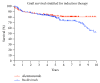
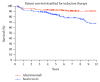
Alemtuzumab, an anti-CD52 T-cell and B-cell depleting monoclonal antibody, is established for induction therapy in renal transplantation (KTx). We herein provide a comparative analysis between alemtuzumab and basiliximab induction therapy and correlate lymphocyte depletion and recovery with the clinical course after KTx. This is a single center retrospective analysis of 225 patients/consecutive kidney transplantations treated with alemtuzumab for lymphocyte depletion and 205 recipients treated with basiliximab. Mean lymphocyte counts were 22.8 ± 9.41% before Tx and 2.61 ± 3.11% between week 1 and week 3 in the alemtuzumab group and 23.77 ± 10.42% before Tx and 13.92 ± 8.20% in the basiliximab group. Delayed graft function (DGF), cytomegalovirus (CMV) status, and recipient age showed a significant correlation with lymphocyte counts in the alemtuzumab group only. The outcome was read in reference to the velocity of lymphocyte recovery and in comparison to the control group. Lymphocyte counts early after transplantation, following alemtuzumab treatment, could be identified as a predictive factor for kidney function early after transplantation. A detailed analysis of phenotype and function of lymphocytes after alemtuzumab induction together with a correlation with the clinical course is warranted.
Figures
Similar articles
-
An analysis of lymphocyte phenotype after steroid avoidance with either alemtuzumab or basiliximab induction in renal transplantation.Am J Transplant. 2012 Apr;12(4):919-31. doi: 10.1111/j.1600-6143.2011.03891.x. Epub 2012 Mar 5. Am J Transplant. 2012. PMID: 22390816 Clinical Trial.
-
Effect of alemtuzumab or basiliximab induction therapy on graft function and survival of kidneys from donors after cardiac death.Transpl Int. 2009 Oct;22(10):1024-7. doi: 10.1111/j.1432-2277.2009.00910.x. Epub 2009 Jul 13. Transpl Int. 2009. PMID: 19624499 No abstract available.
-
Induction therapy in renal transplant recipients: how convincing is the current evidence?Drugs. 2012 Mar 26;72(5):671-83. doi: 10.2165/11631300-000000000-00000. Drugs. 2012. PMID: 22439670 Review.
-
In kidney transplant patients, alemtuzumab but not basiliximab/low-dose rabbit anti-thymocyte globulin induces B cell depletion and regeneration, which associates with a high incidence of de novo donor-specific anti-HLA antibody development.J Immunol. 2013 Sep 1;191(5):2818-28. doi: 10.4049/jimmunol.1203261. Epub 2013 Aug 2. J Immunol. 2013. PMID: 23913968 Clinical Trial.
-
Selection of induction therapy in kidney transplantation.Transpl Int. 2013 Jul;26(7):662-72. doi: 10.1111/tri.12043. Epub 2012 Dec 31. Transpl Int. 2013. PMID: 23279211 Review.
Cited by
-
The Systemic Immune-Inflammation Index Predicts Clinical Outcomes in Kidney Transplant Recipients.In Vivo. 2020 Nov-Dec;34(6):3349-3360. doi: 10.21873/invivo.12173. In Vivo. 2020. PMID: 33144442 Free PMC article.
-
Good Results with Individually Adapted Long-Term Immunosuppression Following Alemtuzumab Versus ATG Induction Therapy in Combined Kidney-Pancreas Transplantation: A Single-Center Report.Ann Transplant. 2019 Jan 25;24:52-56. doi: 10.12659/AOT.911712. Ann Transplant. 2019. PMID: 30679414 Free PMC article. Clinical Trial.
References
-
- Margreiter R., Klempnauer J., Neuhaus P., Muehlbacher F., Boesmueller C., Calne R. Y. Alemtuzumab (Campath-1H) and tacrolimus monotherapy after renal transplantation: results of a prospective randomized trial. American Journal of Transplantation. 2008;8(7):1480–1485. doi: 10.1111/j.1600-6143.2008.02273.x. - DOI - PubMed
-
- Ciancio G., Burke G. W., Gaynor J. J., et al. A randomized trial of thymoglobulin vs. alemtuzumab (with lower dose maintenance immunosuppression) vs. daclizumab in renal transplantation at 24 months of follow-up. Clinical Transplantation. 2008;22(2):200–210. doi: 10.1111/j.1399-0012.2007.00774.x. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

