Fresh frozen plasma for cardiovascular surgery
- PMID: 26171897
- PMCID: PMC8406941
- DOI: 10.1002/14651858.CD007614.pub2
Fresh frozen plasma for cardiovascular surgery
Abstract
Background: Fresh frozen plasma (FFP) is a blood component containing procoagulant factors, which is sometimes used in cardiovascular surgery with the aim of reducing the risk of bleeding. The purpose of this review is to assess the risk of mortality for patients undergoing cardiovascular surgery who receive FFP.
Objectives: To evaluate the risk to benefit ratio of FFP transfusion in cardiovascular surgery for the treatment of bleeding patients or for prophylaxis against bleeding.
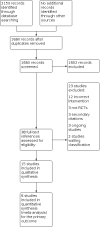
Search methods: We searched 11 bibliographic databases and four ongoing trials databases including the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 3, 2015), MEDLINE (OvidSP, 1946 to 21 April 2015), EMBASE (OvidSP, 1974 to 21 April 2015), PubMed (e-publications only: searched 21 April 2015), ClinicalTrials.gov, World Health Organization (WHO) ICTRP and the ISRCTN Register (searched 21 April 2015). We also searched the references of all identified trials and relevant review articles. We did not limit the searches by language or publication status.
Selection criteria: We included randomised controlled trials in patients undergoing major cardiac or vascular surgery who were allocated to a FFP group or a comparator (no plasma or an active comparator, either clinical plasma (any type) or a plasma-derived blood product). We included participants of any age (neonates, children and adults). We excluded studies of plasmapheresis and plasma exchange.
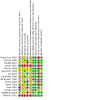
Data collection and analysis: Two authors screened all electronically derived citations and abstracts of papers identified by the review search strategy. Two authors assessed risk of bias in the included studies and extracted data independently. We took care to note whether FFP was used therapeutically or prophylactically within each trial.
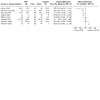
Main results: We included 15 trials, with a total of 755 participants for analysis in the review. Fourteen trials compared prophylactic use of FFP against no FFP. One study compared therapeutic use of two types of plasma. The timing of intervention varied, including FFP transfusion at the time of heparin neutralisation and stopping cardiopulmonary bypass (CPB) (seven trials), with CPB priming (four trials), after anaesthesia induction (one trial) and postoperatively (two trials). Twelve trials excluded patients having emergency surgery and nine excluded patients with coagulopathies.Overall the trials were small, with only four reporting an a priori sample size calculation. No trial was powered to determine changes in mortality as a primary outcome. There was either high risk of bias, or unclear risk, in the majority of trials included in this review.There was no difference in the number of deaths between the intervention arms in the six trials (with 287 patients) reporting mortality (very low quality evidence). There was also no difference in blood loss in the first 24 hours for neonatal/paediatric patients (four trials with 138 patients; low quality evidence): mean difference (MD) -1.46 ml/kg (95% confidence interval (CI) -4.7 to 1.78 ml/kg); or adult patients (one trial with 120 patients): MD -12.00 ml (95% CI -101.16 to 77.16 ml).Transfusion with FFP was inferior to control for preventing patients receiving any red cell transfusion: Peto odds ratio (OR) 2.57 (95% CI 1.30 to 5.08; moderate quality evidence). There was a difference in prothrombin time within two hours of FFP transfusion in eight trials (with 210 patients; moderate quality evidence) favouring the FFP arm: MD -0.71 seconds (95% CI -1.28 to -0.13 seconds). There was no difference in the risk of returning to theatre for reoperation (eight trials with 398 patients; moderate quality evidence): Peto OR 0.81 (95% CI 0.26 to 2.57). Only one included study reported adverse events as an outcome and reported no significant adverse events following FFP transfusion.
Authors' conclusions: This review has found no evidence to support the prophylactic administration of FFP to patients without coagulopathy undergoing elective cardiac surgery. There was insufficient evidence about treatment of patients with coagulopathies or those who are undergoing emergency surgery. There were no reported adverse events attributable to FFP transfusion, although there was a significant increase in the number of patients requiring red cell transfusion who were randomised to FFP. Variability in outcome reporting between trials precluded meta-analysis for many outcomes across all trials, and there was evidence of a high risk of bias in most of the studies. Further adequately powered studies of FFP, or comparable pro-haemostatic agents, are required to assess whether larger reductions in prothrombin time translate into clinical benefits. Overall the evidence from randomised controlled trials for the safety and efficacy of prophylactic transfusion of FFP for cardiac surgery is insufficient.
Conflict of interest statement
MD: None known.
RS: None known.
SB: None known.
CD: None known.
MT: My role as a statistical editor/referee for 4 Cochrane groups (Anaesthesia, Wounds, Breast Cancer, and Sexually Transmitted Infections), and previous editorial work with the Injuries Group, are independent to my involvement in this review. I declare that my involvement here as an author has no related financial relationships.
AM: None known.
IA: None known.
SS: None known.
Figures












Update of
References
References to studies included in this review
Chong Sung 2006 {published data only}
-
- Chong Sung K, Kum Suk P, Mi Ja Y, Kyoung Ok K. Effects of intravascular volume therapy using hydroxyethyl starch (130/0.4) on post-operative bleeding and transfusion requirements in children undergoing cardiac surgery: a randomized clinical trial. Acta Anaesthesiologica Scandinavica 2006;50(1):108-11. - PubMed
-
- Desborough M [per comm]. Effects of intravascular volume therapy using hydroxyethyl starch (130/0.4) on post-operative bleeding and transfusion requirements in children undergoing cardiac surgery: a randomized clinical trial. Email to pissces@medimail.co.kr and kimcs@snu.ac.kr for further information 11 December 2014 No reply. - PubMed
Consten 1996 {published data only}
-
- Consten EC, Henny CP, Eijsman L, Dongelmans DA, Oers MH. The routine use of fresh frozen plasma in operations with cardiopulmonary bypass is not justified. Journal of Thoracic and Cardiovascular Surgery 1996;112(1):162-7. - PubMed
Kanbak 2011 {published and unpublished data}
-
- Desborough M [per comm]. Peroperative effects of fresh frozen plasma and antithrombin III on heparin sensitivity and coagulation during nitroglycerine infusion in coronary artery bypass surgery. Email to baharoc@selcuk.edu.tr 11 December 2014 Reply with information on mortality and number of patients who underwent any transfusion on 15 January 2015.
-
- Kanbak M, Oc B, Salman MA, Ocal T, Oc M. Peroperative effects of fresh frozen plasma and antithrombin III on heparin sensitivity and coagulation during nitroglycerine infusion in coronary artery bypass surgery. Blood Coagulation & Fibrinolysis 2011;22(7):593-9. - PubMed
Kasper 2001 {published data only}
-
- Kasper SM, Giesecke T, Limpers P, Sabatowski R, Mehlhorn U, Diefenbach C. Failure of autologous fresh frozen plasma to reduce blood loss and transfusion requirements in coronary artery bypass surgery. Anesthesiology 2001;95(1):81-6. - PubMed
Kyoung 2004 {published data only}
-
- Desborough M [per comm]. Effects of intravascular volume therapy with a hydroxyethal starch [HES 130/0.4] on blood coagulation in children undergoing cardiac surgery. Email to pissces@medimail.co.kr and kimcs@snu.ac.kr for further information 11 December 2014 No reply.
-
- Kyoung K, Sun S, Sung KC. Effects of intravascular volume therapy with a hydroxyethal starch [HES 130/0.4] on blood coagulation in children undergoing cardiac surgery [심장수술을 시행하는 환아에서 혈장증량제로 사용한 히드록시에칠스타치가 혈액응고에 미치는 영향]. Korean Journal of Anaesthesiology 2004;47(3):379-84.
Långström 2008 {published data only}
-
- Långström S, Rautiainen P, Mildh L, Peltola K, Wartiovaara-Kautto U, Heikinheimo M et al. Fresh frozen plasma does not reduce in vivo thrombin formation after neonatal cardiopulmonary bypass surgery. Thrombosis and Haemostasis 2008;100(6):1207-8. - PubMed
Lee 2013 {published data only}
Loeffelbein 2008 {published data only}
-
- Loeffelbein F, Zirell U, Benk C, Schlensak C, Dittrich S. High colloid oncotic pressure priming of cardiopulmonary bypass in neonates and infants: implications on haemofiltration, weight gain and renal function. European Journal of Cardio-Thoracic Surgery 2008;34(3):648-52. - PubMed
Martinowitz 1990 {published data only}
-
- Martinowitz U, Goor DA, Ramot B, Mohr R. Is transfusion of fresh plasma after cardiac operations indicated? Journal of Thoracic and Cardiovascular Surgery 1990;100(1):92-8. - PubMed
McCall 2004 {published and unpublished data}
-
- Desborough M [per comm]. Fresh frozen plasma in the pediatric pump prime: a prospective, randomized trial. Email to bradlesm@musc.edu 11 December 2014 Reply with information on mortality and number of patients who underwent any transfusion on 12 December 2014.
-
- McCall MM, Blackwell MM, Smyre JT, Sistino JJ, Acsell JR, Dorman BH et al. Fresh frozen plasma in the pediatric pump prime: a prospective, randomized trial. Annals of Thoracic Surgery 2004;77(3):983-7. - PubMed
Oliver 2003 {published and unpublished data}
-
- Desborough M [per comm]. Blood loss in infants and children for open heart operations: albumin 5% versus fresh-frozen plasma in the prime. Email to oliver.william@mayo.edu for further information 11 December 2014 Reply with information on number of patients who underwent any transfusion on 16 January 2015.
-
- Oliver WC Jr, Beynen FM, Nuttall GA, Schroeder DR, Ereth MH, Dearani JA et al. Blood loss in infants and children for open heart operations: albumin 5% versus fresh-frozen plasma in the prime. Annals of Thoracic Surgery 2003;75(5):1506-12. - PubMed
Snow 1982 {published data only}
-
- Snow N, Lucas AE. Prophylactic fresh frozen plasma administration: failure to reduce blood loss or transfusion requirements after routine cardiopulmonary bypass. Journal of Extra-Corporeal Technology 1982;14(4):400-4.
Trimble 1964 {published data only}
-
- Trimble AS, Osborn JJ, Kerth WJ, Gerbode F. The prophylactic use of fresh frozen plasma after extracorporeal circulation. Journal of Thoracic and Cardiovascular Surgery 1964;48(2):314-6. - PubMed
Tølløfsrud 2003 {published data only}
-
- Desborough M [per comm]. Universal solvent/detergent-treated fresh frozen plasma (Uniplas--rationale and clinical properties). Email to bjarte.solheim@rikshospitalet.no for further information 11 December 2014 Email address no longer in use.
-
- Noddeland H, Töllöfsrud S, Svennevig J, Bentsen G, Brosstad F, Solheim B. Universal solvent/detergent-treated fresh frozen plasma (Uniplas--rationale and clinical properties). Thrombosis Research 2002;107(Suppl 1):S33-7. - PubMed
-
- Tølløfsrud S, Noddeland H, Svennevig JL, Bentsen G, Mollnes TE, Solheim BG. Universal fresh frozen plasma (Uniplas): a safe product in open-heart surgery. Intensive Care Medicine 2003;29(10):1736-43. - PubMed
Wilhelmi 2001 {published data only}
-
- Wilhelmi M, Franke U, Cohnert T, Weber P, Kaukemüller J, Fischer S et al. Coronary artery bypass grafting surgery without the routine application of blood products: is it feasible? European Journal of Cardiothoracic Surgery 2001;19(5):657-61. - PubMed
References to studies excluded from this review
Armellin 2001 {published data only}
-
- Armellin G, Sorbara C, Bonato R, Pittarello D, Dal Cero P, Giron G. Intraoperative plasmapheresis in cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 1997;11(1):13-7. - PubMed
Bilgin 2011 {published data only}
-
- Bilgin YM, de Watering LM, Versteegh MI, Oers MH, Vamvakas EC, Brand A. Postoperative complications associated with transfusion of platelets and plasma in cardiac surgery. Transfusion 2011;51(12):2603-10. - PubMed
Boldt 1989 {published and unpublished data}
-
- Boldt J, Kling D, Bormann M, Zuge M, Hempelmann G. Homologes Frischplasma in der Herzchirurgie. Anaesthesist 1989;38:53-9. - PubMed
Boldt 1990 {published data only}
-
- Boldt J, VonBormann B, Kling D, Jacobi M, Moosdorf R, Hempelmann G. Preoperative plasmapheresis in patients undergoing cardiac surgery procedures. Anesthesiology 1990;72(2):282-8. - PubMed
Boldt 1993 {published data only}
-
- Boldt J, Zickmann B, Ballesteros M, Oehmke S, Stertmann F, Hempelmann G. Influence of acute preoperative plasmapheresis on platelet function in cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 1993;7(1):4-9. - PubMed
Chapanduka 2002 {published data only}
-
- Chapanduka ZC, Fernandes-Costa FJTD, Rochat C, Blyth DF. Comparative safety and efficacy of Bioplasma FDP versus single-donor fresh-dried plasma in cardiopulmonary bypass patients. South African Medical Journal 2002;92(5):356-7. - PubMed
Demeyere 2010 {published data only}
-
- Demeyere R, Arnout J, Strengers P. Prothrombin complex concentrate versus fresh frozen plasma in patients on oral anticoagulant therapy undergoing cardiac surgery: a randomized study. In: Critical Care. Vol. 10 (Suppl 1). 2006:P233.
-
- Demeyere R, Gillardin S, Arnout J, Strengers PF. Comparison of fresh frozen plasma and prothrombin complex concentrate for the reversal of oral anticoagulants in patients undergoing cardiopulmonary bypass surgery: a randomized study. Vox Sanguinis 2010;99(3):251-60. - PubMed
Frenzel 2008 {published and unpublished data}
-
- Frenzel W. Study of OCTAPLEX and FFP in patients under Vitamin K therapy antagonist needing urgent surgery or invasive procedures. https://clinicaltrials.gov/ct2/show/NCT00618098 2008. [NCT00618098]
Haubelt 2002 {published data only}
-
- Haubelt H, Blome M, Kiessling AH, Isgro F, Bach J, Saggau W et al. Effects of solvent/detergent-treated plasma and fresh-frozen plasma on haemostasis and fibrinolysis in complex coagulopathy following open-heart surgery. Vox Sanguinis 2002;82:9-14. - PubMed
Hertfelder 1992 {published data only}
-
- Hertfelder HJ, Papov-Cenic S, Urban A, Dusterwald M, Brecher AM. Activation of hemostasis by transfusions of whole blood or blood components in open heart surgery of children. In: Annals of Hematology. Vol. 64. 1992:A32.
Lancé 2012 {published data only}
-
- Lance MD, Ninivaggi M, Schols SEM, Feijge MAH, Oehrl SK, Kuiper GJAJM et al. Perioperative dilutional coagulopathy treated with fresh frozen plasma and fibrinogen concentrate: a prospective randomized intervention trial. Vox Sanguinis 2012;103(1):25-34. - PubMed
Menges 2006 {published data only}
-
- Menges T, Wagner RM, Welters I, Ruwoldt R, Boldt J, Hempelmann G. The role of the protein C-thrombomodulin system and fibrinolysis during cardiovascular surgery: influence of acute preoperative plasmapheresis. Journal of Cardiothoracic and Vascular Anesthesia 1996;10(4):482-9. - PubMed
Safwat 2002 {published data only}
-
- Safwat AM, Bush R, Prevec W, Reitan JA. Intraoperative use of platelet-plasmapheresis in vascular surgery. Journal of Clinical Anesthesia 2002;14(1):10-4. - PubMed
von Sommoggy 1990 {published data only}
-
- Sommoggly S, Fraunhofer J, Jelen-Esselborn S, Stemberger A. Coagulation changes during aortofemoral bifurcation bypass operations: is plasma substitution possible with hydroxyethyl starch (HES) only? [Gerinnungsveränderungen bei aortofemoralem Bifurkationsbypass: ist eine Volumen- und Plasmasubstitution mit Hydroxyäthylstärke allein möglich?]. Anaesthesist 1990;39:353-60. - PubMed
Yiu 2006 {published data only}
-
- Yiu K, Siu C, Jim M, Tse H, Fan K, Chau M et al. Comparison of the efficacy and safety profiles of intravenous vitamin K and fresh frozen plasma as treatment of warfarin-related over-anticoagulation in patients with mechanical heart valves. American Journal of Cardiology 2006;97(3):409-11. - PubMed
References to studies awaiting assessment
Miao 2014 {published data only}
-
- Miao X, Liu J, Zhao M, Cui Y, Feng Z, Zhao J et al. The influence of cardiopulmonary bypass priming without FFP on postoperative coagulation and recovery in pediatric patients with cyanotic congenital heart disease. European Journal of Paediatrics 2014;173(11):1437-43. - PubMed
Miao 2015 {published data only}
-
- Liu J, Feng Z, Zhao J, Miao X, Long C. The impact of priming without plasma during cardiopulmonary bypass on postoperative coagulation and clinical recovery in infants underwent cardiac surgery. In: Cardiology (Switzerland). Vol. 129. 2014:112-3.
-
- Miao X, Liu J, Zhao M, Cui Y, Feng Z, Zhao J et al. Evidence-based use of FFP: the influence of a priming strategy without FFP during CPB on postoperative coagulation and recovery in pediatric patients. Perfusion 2015;30(2):140-7. - PubMed
References to ongoing studies
ACTRN12613001279718 {published data only}
-
- Prothrombinex-VF vs fresh frozen plasma for the treatment of bleeding post-cardiopulmonary bypass. https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365300. [ACTRN12613001279718]
EudraCT: 2009‐016709‐41 {published data only}
-
- Coagulopathy during surgery for the repair of extent 4 thoraco-abdominal aortic aneurysms - feasibility study of the use of fibrinogen concentrate by infusion in place of fresh frozen plasma. https://www.clinicaltrialsregister.eu/ctr-search/search?query=2009-01670.... [2009-016709-41]
EudraCT: 2014‐000452‐28 {published data only}
-
- Vasculopathic Injury and Plasma as Endothelial Rescue - OCTAplas Trial (VIPER-OCTA). https://www.clinicaltrialsregister.eu/ctr-search/search?query=2014-00045.... [2014-000452-28]
Additional references
Andrzejewski 2005
-
- Andrzejewski C, Popovsky MA. Transfusion-associated adverse pulmonary sequelae: widening our perspective. Transfusion 2005;45(7):1048-50. - PubMed
BCSH Guidelines 2004
-
- O'Shaughnessy DF, Atterbury C, Bolton Maggs P, Murphy M, Thomas D, Yates S et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. British Journal of Haematology 2004;126(1):11-28. - PubMed
Bevan 1999
-
- Bevan DH. Cardiac bypass haemostasis: putting blood through the mill. British Journal of Haematology 1999;104(2):208-19. - PubMed
Bjursten 2013
-
- Bjursten H, Dardashti A, Ederoth P, Brondén B, Algotsson L. Increased long-term mortality with plasma transfusion after coronary artery bypass surgery. Intensive Care Medicine 2013;39(3):437-44. - PubMed
Boccia 2009
-
- Boccia S. PRISMA: an attempt to improve standards for reporting systematic review and meta-analysis. Journal of Public Health 2009;6(4):352-3.
CMA Guidelines 1997
-
- Canadian Medical Association Expert Working Group. Guidelines for red blood cell and plasma transfusion for adults and children. Canadian Medical Association Journal 1997;156(11 Suppl):S1-S24.
Davidson 2014
-
- Davidson S. State of the art: how I manage coagulopathy in cardiac surgery patients. British Journal of Haematology 2014;164(6):779-89. - PubMed
Desborough 2012
-
- Desborough M, Stanworth S. Plasma transfusion for bedside, radiologically guided, and operating room invasive procedures. Transfusion 2012;52(Suppl 1):20S-9S. - PubMed
Doussau 2014
-
- Doussau A, Perez P, Puntous M, Calderon J, Jeanne M, Germain C et al. Fresh-frozen plasma transfusion did not reduce 30-day mortality in patients undergoing cardiopulmonary bypass cardiac surgery with excessive bleeding: the PLASMACARD multicenter cohort study. Transfusion 2014;54(4):1114-24. - PubMed
GRADEpro 2014 [Computer program]
-
- McMaster University GRADEpro [Computer program on www.gradepro.org]. Version [3rd June 2015]. McMaster University, 2014.
Hartmann 2006
-
- Hartmann M, Sucker C, Boehm O, Koch A, Loer S, Zacharowski K. Effects of cardiac surgery on hemostasis. Transfusion Medicine Reviews 2006;20(3):230-41. - PubMed
Higgins 2011a
-
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Higgins 2011b
-
- Higgins JPT, Deeks JJ (editors). Chapter 7: Selecting studies and collecting data. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Juni 2001
Khan 2007
-
- Khan H, Belsher J, Yilmaz M, Afessa B, Winters JL, Moore SB et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest 2007;131(5):1308-14. - PubMed
Langendam 2013
Lundberg 1994
-
- Lundberg GD. Practice parameter for the use of fresh-frozen plasma, cryoprecipitate, and platelets. Fresh-frozen Plasma, Cryoprecipitate, and Platelets Administration Practice Guidelines Development Task Force of the College of American Pathologists. JAMA 1994;271(10):777-81. - PubMed
MacLennan 2006
-
- MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. Journal of Trauma 2006;60(6 Suppl):S46-50. - PubMed
Narick 2012
-
- Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion 2012;52(1):160-5. - PubMed
Palo 2006
-
- Palo R, Capraro L, Hovilehto S, Koivuranta M, Krusius T, Loponen E et al. Population-based audit of fresh-frozen plasma transfusion practices. Transfusion 2006;46(11):1921-5. - PubMed
Pamphilon 2000
-
- Pamphilon D. Viral inactivation of fresh frozen plasma. British Journal of Haematology 2000;109(4):680-93. - PubMed
Pelletier 2006
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roseff 2002
-
- Roseff SD, Luban NL, Manno CS. Guidelines for assessing appropriateness of pediatric transfusion. Transfusion 2002;42(11):1398-413. - PubMed
SHOT 2014
-
- Serious Hazards of Transfusion Steering Group. Annual SHOT Report 2013. Available at http://www.shotuk.org (accessed 28 April 2015) 2014.
Skeate 2007
-
- Skeate RC, Eastlund T. Distinguishing between transfusion related acute lung injury and transfusion associated circulatory overload. Current Opinion in Hematology 2007;14(6):682-7. - PubMed
Stanworth 2011
-
- Stanworth SJ, Grant-Casey J, Lowe D, Laffan M, New H, Murphy MF et al. The use of fresh-frozen plasma in England: high levels of inappropriate use in adults and children. Transfusion 2011;51(1):62-70. - PubMed
Vlaar 2013
-
- Vlaar AP, Juffermans NP. Transfusion-related acute lung injury: a clinical review. Lancet 2013;382(9896):984-94. - PubMed
Vuylsteke 2011
-
- Vuylsteke A, Pagel C, Gerrard C, Reddy B, Nashef S, Aldam P et al. The Papworth Bleeding Risk Score: a stratification scheme for identifying cardiac surgery patients at risk of excessive early postoperative bleeding. European Journal of Cardiothoracic Surgery 2011;39(6):924-30. - PubMed
Wallis 2004
-
- Wallis JP, Dzik S. Is fresh frozen plasma overtransfused in the United States? Transfusion 2004;44(11):1674-5. - PubMed
Wise 2013
-
- Wise J. Boldt: the great pretender. BMJ 2013;346:f1738. - PubMed
Yang 2012
-
- Yang L, Stanworth S, Hopewell S, Doree C, Murphy M. Is fresh-frozen plasma clinically effective? An update of a systematic review of randomized controlled trials. Transfusion 2012;52(8):1673-86. - PubMed
References to other published versions of this review
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

