Oral zinc for the prevention of hyperbilirubinaemia in neonates
- PMID: 26171899
- PMCID: PMC7390467
- DOI: 10.1002/14651858.CD008432.pub2
Oral zinc for the prevention of hyperbilirubinaemia in neonates
Abstract
Background: Between 6% and 15% of neonates develop hyperbilirubinaemia requiring treatment. Successful management of neonatal hyperbilirubinaemia relies on prevention and early treatment, with phototherapy being the mainstay of treatment. Oral zinc has been reported to decrease the serum total bilirubin (STB), presumably by decreasing the enterohepatic circulation.
Objectives: To determine the effect of oral zinc supplementation compared to placebo or no treatment on the incidence of hyperbilirubinaemia in neonates during the first week of life and to assess the safety of oral zinc in enrolled neonates.
Search methods: We searched CENTRAL (The Cochrane Library 2014, Issue 1), MEDLINE (1966 to November 30, 2014), and EMBASE (1990 to November 30, 2014).
Selection criteria: Randomised controlled trials were eligible for inclusion if they enrolled neonates (term and preterm) to whom oral zinc, in a dose of 10 to 20 mg/day, was initiated within the first 96 hours of life, for any duration until day seven, compared with no treatment or placebo.
Data collection and analysis: We used the standard methods of The Cochrane Collaboration and its Neonatal Review Group for data collection and analysis.
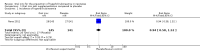
Main results: Only one study met the criteria of inclusion in the review. This study compared oral zinc with placebo. Oral zinc was administered in a dose of 5 mL twice daily from day 2 to day 7 postpartum. The drug was administered into the mouth of the infant by the plastic measure provided with the bottle or with a spoon. Incidence of hyperbilirubinaemia, defined as serum total bilirubin (STB) ≥ 15 mg/dL, was similar between groups (N = 286; risk ratio (RR) 0.94, 95% confidence interval (CI) 0.58 to 1.52). Mean STB levels, mg/dL, at 72 ± 12 hours were comparable in both the groups (N = 286; mean difference (MD) -0.20; 95% CI -1.03 to 0.63). Although the duration of phototherapy in the zinc group was significantly shorter compared to the placebo group (N = 286; MD -12.80, 95% CI -16.93 to -8.67), the incidence of need for phototherapy was comparable across both the groups (N = 286; RR 1.20; 95% CI 0.66 to 2.18). Incidences of side effects like vomiting (N = 286; RR 0.65, 95% CI 0.19 to 2.25), diarrhoea (N = 286; RR 2.92, 95% CI 0.31 to 27.71), and rash (N = 286; RR 2.92, 95% CI 0.12 to 71.03) were found to be rare and statistically comparable between groups.
Authors' conclusions: The limited evidence available has not shown that oral zinc supplementation given to infants up to one week old reduces the incidence of hyperbilirubinaemia or need for phototherapy.
Conflict of interest statement
No conflict of interest.
Figures
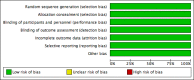






Update of
- doi: 10.1002/14651858.CD008432
Similar articles
-
Fluid supplementation for neonatal unconjugated hyperbilirubinaemia.Cochrane Database Syst Rev. 2017 Aug 1;8(8):CD011891. doi: 10.1002/14651858.CD011891.pub2. Cochrane Database Syst Rev. 2017. PMID: 28762235 Free PMC article.
-
Periodic change of body position under phototherapy in term and preterm neonates with hyperbilirubinaemia.Cochrane Database Syst Rev. 2022 Mar 2;3(3):CD011997. doi: 10.1002/14651858.CD011997.pub2. Cochrane Database Syst Rev. 2022. PMID: 35235686 Free PMC article.
-
Clofibrate in combination with phototherapy for unconjugated neonatal hyperbilirubinaemia.Cochrane Database Syst Rev. 2012 Dec 12;12(12):CD009017. doi: 10.1002/14651858.CD009017.pub2. Cochrane Database Syst Rev. 2012. PMID: 23235669 Free PMC article.
-
Sertindole for schizophrenia.Cochrane Database Syst Rev. 2005 Jul 20;2005(3):CD001715. doi: 10.1002/14651858.CD001715.pub2. Cochrane Database Syst Rev. 2005. PMID: 16034864 Free PMC article.
-
Light-emitting diode phototherapy for unconjugated hyperbilirubinaemia in neonates.Cochrane Database Syst Rev. 2011 Dec 7;2011(12):CD007969. doi: 10.1002/14651858.CD007969.pub2. Cochrane Database Syst Rev. 2011. PMID: 22161417 Free PMC article.
Cited by
-
International guidelines regarding the role of IVIG in the management of Rh- and ABO-mediated haemolytic disease of the newborn.Br J Haematol. 2022 Jul;198(1):183-195. doi: 10.1111/bjh.18170. Epub 2022 Apr 12. Br J Haematol. 2022. PMID: 35415922 Free PMC article.
-
Enteral zinc supplementation for prevention of morbidity and mortality in preterm neonates.Cochrane Database Syst Rev. 2021 Mar 12;3(3):CD012797. doi: 10.1002/14651858.CD012797.pub2. Cochrane Database Syst Rev. 2021. PMID: 33710626 Free PMC article.
References
References to studies included in this review
Rana 2011 {published data only}
-
- Rana N, Mishra S, Bhatnagar S, Paul V, Deorari AK, Agarwal R. Efficacy of zinc in reducing hyperbilirubinemia among at‐risk neonates: a randomized, double‐blind placebo‐controlled trial. Indian Journal Pediatrics 2011;78(9):1073‐8. [PUBMED: 21455724] - PubMed
Additional references
American Academy of Pediatrics 2004
-
- American Academy of Pediatrics Subcommittee on Hyperbilirubinemia. Management of hyperbilirubinemia in the newborn infant 35 or more weeks of gestation. Pediatrics 2004;114(1):297–316. - PubMed
Bloomer 1976
-
- Bloomer JR, Zaccaria J. Effect of graded bilirubin load on bilirubin transport by perfused rat liver. American Journal of Physiology 1976;230(3):736‐42. [PUBMED: 1266978] - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Madan 2005
-
- Madan A, McMahon JR, Stevenson DK. Neonatal hyperbilirubinemia. In: Taeusch HW, Ballard RA, Gleason CA editor(s). Textbook of Neonatology. 8th Edition. Philadelphia: Elsevier Saunders, 2005:1226‐56.
Maisels 1988
-
- Maisels MJ, Gifford K, Antle CE, Leib GR. Jaundice in the healthy newborn infant: a new approach to an old problem. Pediatrics 1988;81(4):505‐11. [PUBMED: 3353184] - PubMed
Maisels 1992
-
- Maisels MJ. Neonatal jaundice. In: Sinclair JC, Bracken MB editor(s). Effective Care of the Newborn Infant. Oxford: Oxford University Press, 1992:507‐19.
Méndez‐Sánchez 2001
-
- Méndez‐Sánchez N, Roldán‐Valadez E, Flores MA, Cárdenas‐Vázquez R, Uribe M. Zinc salts precipitate unconjugated bilirubin in vitro and inhibit enterohepatic cycling of bilirubin in hamsters. European Journal of Clinical Investigation 2001;31(9):773‐80. [PUBMED: 11589719] - PubMed
Méndez‐Sánchez 2002
-
- Méndez‐Sánchez N, Martínez M, González V, Roldán‐Valadez E, Flores MA, Uribe M. Zinc sulphate inhibits the enterohepatic cycling of unconjugated bilirubin in subjects with Gilbert's syndrome. Annals of Hepatology 2002;1(1):40‐3. [PUBMED: 15114295] - PubMed
Narang 1997
-
- Narang A, Gathwala G, Kumar P. Neonatal jaundice: an analysis of 551 cases. Indian Pediatrics 1997;34(5):429‐32. [PUBMED: 9332119] - PubMed
Odell 1983
-
- Odell GB, Gutcher GR, Whitington PF, Yang G. Enteral administration of agar as an effective adjunct to phototherapy of neonatal hyperbilirubinemia. Pediatric Research 1983;17(10):810‐4. [PUBMED: 6415606] - PubMed
Shapiro 2003
-
- Shapiro SM. Bilirubin toxicity in the developing nervous system. Pediatric Neurology 2003;29(5):410‐21. [PUBMED: 14684236] - PubMed
Suresh 2003
Vitek 2005
-
- Vitek L, Muchova L, Zelenka J, Zadinova M, Malina J. The effect of zinc salts on serum bilirubin levels in hyperbilirubinemic rats. Journal of Pediatric Gastroenterology and Nutrition 2005;40(2):135‐40. [PUBMED: 15699685] - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

