Long-acting FSH versus daily FSH for women undergoing assisted reproduction
- PMID: 26171903
- PMCID: PMC10415736
- DOI: 10.1002/14651858.CD009577.pub3
Long-acting FSH versus daily FSH for women undergoing assisted reproduction
Abstract
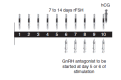
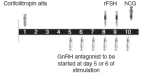
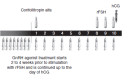
Background: Assisted reproduction techniques (ART), such as in vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI), can help subfertile couples to create a family. It is necessary to induce multiple follicles, which is achieved by follicle stimulating hormone (FSH) injections. Current treatment regimens prescribe daily injections of FSH (urinary FSH either with or without luteinizing hormone (LH) injections or recombinant FSH (rFSH)).Recombinant DNA technologies have produced a new recombinant molecule which is a long-acting FSH, named corifollitropin alfa (Elonva) or FSH-CTP. A single dose of long-acting FSH is able to keep the circulating FSH level above the threshold necessary to support multi-follicular growth for an entire week. The optimal dose of long-acting FSH is still being determined. A single injection of long-acting FSH can replace seven daily FSH injections during the first week of controlled ovarian stimulation (COS) and can make assisted reproduction more patient friendly.
Objectives: To compare the effectiveness of long-acting FSH versus daily FSH in terms of pregnancy and safety outcomes in women undergoing IVF or ICSI treatment cycles.
Search methods: We searched the following electronic databases, trial registers and websites from inception to June 2015: the Cochrane Central Register of Controlled Trials (CENTRAL), the Cochrane Menstrual Disorders and Subfertility Group (MDSG) Specialized Register, MEDLINE, EMBASE, PsycINFO, CINAHL, electronic trial registers for ongoing and registered trials, citation indexes, conference abstracts in the ISI Web of Knowledge, LILACS, Clinical Study Results (for clinical trial results of marketed pharmaceuticals), PubMed and OpenSIGLE. We also carried out handsearches.
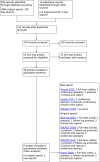
Selection criteria: We included all randomised controlled trials (RCTs) comparing long-acting FSH versus daily FSH in women who were part of a couple with subfertility and undertaking IVF or ICSI treatment cycles with a GnRH antagonist or agonist protocol.
Data collection and analysis: Two review authors independently performed study selection, data extraction and assessment of risk of bias. We contacted trial authors in cases of missing data. We calculated risk ratios for each outcome, and our primary outcomes were live birth rate and ovarian hyperstimulation syndrome (OHSS) rate. Our secondary outcomes were ongoing pregnancy rate, clinical pregnancy rate, multiple pregnancy rate, miscarriage rate, any other adverse event (including ectopic pregnancy, congenital malformations, drug side effects and infection) and patient satisfaction with the treatment. Trials reported all outcomes, except patient satisfaction with the treatment.
Main results: We included six RCTs with a total of 3753 participants and we graded the quality of the included studies as moderate. All studies included women with an indication for COS as part of an IVF/ICSI cycle with age ranging from 18 to 41 years. A comparison of long-acting FSH versus daily FSH did not show evidence of difference in effect on overall live birth rate (Risk ratio (RR) 0.95, 95% confidence interval (CI) 0.84 to 1.07; 2363 participants, eight studies; I² statistic = 44%) or OHSS (RR 1.00, 95% CI 0.74 to 1.37; 3753 participants, nine studies; I² statistic = 0%). We compared subgroups by dose of long-acting FSH. There was evidence of reduced live birth rate in women who received lower doses (60 to 120 μg) of long-acting FSH compared to daily FSH (RR 0.70, 95% CI 0.52 to 0.93; 645 participants, three studies; I² statistic = 0%). There was no evidence a difference between the groups in live births in the medium dose (150 to 180 μg) subgroup (RR 1.03, 95% CI 0.90 to 1.18; 1685 participants, four studies; I² statistic = 6%). There was no evidence of a difference between the groups in the clinical pregnancy rate (any dose), ongoing pregnancy rate (any dose), multiple pregnancy rate (any dose), miscarriage rate (low or medium dose), ectopic pregnancy rate (any dose), congenital malformation rate, congenital malformation rate; major or minor (low or medium dose).
Authors' conclusions: The use of a medium dose (150 to 180 μg) of long-acting FSH is a safe treatment option and equally effective compared to daily FSH in women with unexplained subfertility. There was evidence of reduced live birth rate in women receiving a low dose (60 to 120 μg) of long-acting FSH compared to daily FSH. Further research is needed to determine whether long-acting FSH is safe and effective for use in hyper- or poor responders and in women with all causes of subfertility.
Conflict of interest statement
The authors have no interests to declare.
Figures














Update of
-
Long-acting FSH versus daily FSH for women undergoing assisted reproduction.Cochrane Database Syst Rev. 2012 Jun 13;(6):CD009577. doi: 10.1002/14651858.CD009577.pub2. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2015 Jul 14;(7):CD009577. doi: 10.1002/14651858.CD009577.pub3. PMID: 22696386 Updated.
References
References to studies included in this review
Devroey 2004 {published and unpublished data}
-
- Devroey P, Fauser BC, Platteau P, Beckers NG, Dhont M, Mannaerts BM. Induction of multiple follicular development by a single dose of long‐acting recombinant follicle‐stimulating hormone (FSH‐CTP, corifollitropin alfa) for controlled ovarian stimulation before in vitro fertilization. Journal of Clinical Endocrinology and Metabolism May;89(5):2062‐70. - PubMed
-
- NCT00702195. Pregnancy and neonatal follow‐up of ongoing pregnancies established during phase II clinical trials for the development of Org 36286 (corifollitropin alfa). clinicaltrials.gov/show/NCT00702195 (accessed 18 June 2008).
-
- NCT00702195. Pregnancy and neonatal follow‐up of ongoing pregnancies established during phase II clinical trials for the development of Org 36286 (corifollitropin alfa). www.clinicalstudyresults.org (accessed 27 May 2009).
-
- NCT00702806. A phase III, open‐label, prospective, randomized, comparative clinical trial to investigate the appropriate dose of a single injection of Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or IVF/ICSI. clinicaltrials.gov/show/NCT00702806 (accessed 18 June 2008).
-
- NCT00702806. A phase III, open‐label, prospective, randomized, comparative clinical trial to investigate the appropriate dose of a single injection of Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or IVF/ICSI. www.clinicalstudyresults.org (accessed 11 February 2008).
ENGAGE 2009 {published and unpublished data}
-
- Behr B, Heijnen E. Corifollitropin alfa versus recombinant FSH treatment for COS in a GnRH antagonist protocol: equal efficacy in terms of oocyte and embryo quality. Human Reproduction, European Society of Human Reproduction and Embryology, ESHRE 25th Annual Meeting; 2009 June 28‐July 1 2009; Amsterdam, The Netherlands. 2009.
-
- Bonduelle M, Mannaerts B, Leader A, Bergh C, Passier D, Devroey P. Prospective follow‐up of 838 fetuses conceived after ovarian stimulation with corifollitropin alfa: comparative and overall neonatal outcome. Human Reproduction 2012;27(7):2177‐85. - PubMed
-
- Boostanfar R, Devroey P, Oberye J, Mannaerts B. No difference in the cumulative pregnancy rates or live birth rates when comparing corifollitropin alfa with daily rFSH treatment for COS. Human Reproduction. Abstracts of the 26th Annual Meeting of ESHRE; 2010 June 27‐30; Rome. 2010; Vol. 25 Suppl 1(6):i47 Abstract no. O‐119.
-
- Boostanfar R, Mannarts B, Witjes H, Devroey P. International differences in IVF live birth rates and ongoing pregnancy rates following ovarian stimulation with corifollitropin alfa or recombinant FSH. Fertility and Sterility, Annual Meeting of the American Society for Reproductive Medicine. 2011 Oct 15‐19; Orlando 2011;96(Suppl 3):174.
-
- Devroey P, Boostanfar R, Koper NP, Mannaerts BM, Ijzerman‐Boon PC, Fauser BC, ENGAGE Investigators. Erratum: A double‐blind, non‐inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Human Reproduction 2014;29(5):1116‐1120. - PMC - PubMed
ENSURE 2010 {published and unpublished data}
-
- Bonduelle M, Mannaerts B, Leader A, Bergh C, Passier D, Devroey P. Prospective follow‐up of 838 fetuses conceived after ovarian stimulation with corifollitropin alfa: comparative and overall neonatal outcome. Human Reproduction 2012;27(7):2177‐85. - PubMed
-
- Corifollitropin alfa Ensure Study Group: Obruca A, Schenk M, Tews G, Mardesic T, Mrazek M, Meinertz H, et al. Corifollitropin alfa for ovarian stimulation in IVF: a randomized trial in lower‐body‐weight women. Reproductive BioMedicine Online 2010;21(1):66‐76. - PubMed
-
- Hillenjso T, Mauw E. A comparison of corifollitropin vs. recombinant FSH in a GnRH antagonist protocol for controlled ovarian stimulation in women weighing 60 kg or less. Human Reproduction. European Society of Human Reproduction and Embryology, ESHRE 25th Annual Meeting; 2009 June 28‐July 1; Amsterdam. 2009; Vol. 24 Suppl 1:i2.
-
- NCT00702546. Follow‐up protocol to collect the outcome of frozen‐thawed embryo transfer cycles after cryopreservation of embryos in clinical trial 107012. clinicaltrials.gov/show/NCT00702546 (accessed 18 June 2008).
-
- NCT00702546. Follow‐up protocol to collect the outcome of frozen‐thawed embryo transfer cycles after cryopreservation of embryos in clinical trial 107012 (P05711). www.clinicalstudyresults.org (accessed November 2009).
Koper 2008 {published and unpublished data}
-
- Corifollitropin Alfa Dose‐finding Study Group: Abyholm T, Anderson AN, Balen AH, Braat DDM, Devroey P, D'Hooge TH, et al. A randomized dose‐response trial of a single injection of corifollitropin alfa to sustain multifollicular growth during controlled ovarian stimulation. Human Reproduction 2008;23(11):2484‐92. - PubMed
-
- NCT005982088. A phase 2, open label, randomized trial to investigate the dose‐response relationship of a single injection or Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or ICSI. clinicaltrials.gov/show/NCT005982088 (accessed 10 January 2008).
-
- NCT005982088. A phase 2, open label, randomized trial to investigate the dose‐response relationship of a single injection or Org 36286 (corifollitropin alfa) to initiate multiple follicular growth in a controlled ovarian hyperstimulation protocol for IVF or ICSI. www.clinicalstudyresults.org (accessed 11 July 2008).
-
- NCT00702988. Pregnancy and neonatal follow‐up of ongoing pregnancies established during a previous clinical trial for the development of Org 36286 (corifollitropin alfa) (38827/P06056). www.clinicalstudyresults.org (accessed 28 May 2009).
-
- NCT00702988. Pregnancy and neonatal follow‐up of ongoing pregnancies established during clinical trial 38826 for the development of Org 36286 (corifollitropin alfa). clinicaltrials.gov/show/NCT00702988 (accessed 18 June 2008).
Pursue 2012 {published and unpublished data}
-
- Boostanfar R, Mannaerts B, Pang S, Fernandez‐Sanchez M, Witjes H, Devroey P, Engage Investigators. A comparison of live birth rates and cumulative ongoing pregnancy rates between Europe and North America after ovarian stimulation with corifollitropin alfa or recombinant follicle‐stimulating hormone. Fertility and Sterility 2012;97(6):1351‐8. - PubMed
-
- Boostanfar R, Mannaerts B, Witjes H, Devroey P. International differences in IVF live birth rates and cumulative ongoing pregnancy rates following ovarian stimulation with corifollitropin alfa or recombinant FSH. Fertility and Sterility 2011;1:S174. - PubMed
-
- Boostanfar R, Yeko T, Shapiro B, Elbers J, Witjes H, Mannaerts B. A large double‐blind efficacy and safety trial of corifollitropin alfa versus daily recombinant FSH in women 35 to 42 years of age undergoing ovarian stimulation prior to IVF or ICSI (pursue trial). Fertility and Sterility 2012;98(Suppl 1):S34.
-
- NCT01144416. A Phase III, Randomized, Double‐blind, Active‐controlled, Non‐inferiority Trial to Investigate the Efficacy and Safety of a single injection of SCH 900962 (Corifollitropin alfa) to Induce Multifollicular Development for Controlled Ovarian Stimulation (COS) Using Daily Recombinant FSH (recFSH) as a Reference in Women aged 35‐42 Years (P06029). clinicaltrials.gov/show/ NCT01144416 (accessed 11 June 2010).
-
- NCT01144416. Efficacy and safety study of a single injection of SCH 900962 versus daily recFSH injections in women undergoing controlled ovarian stimulation (study P06029 AM1) (PURSUE). clinicaltrials.gov/show/NCT01144416 (accessed 11 November 2013).
Sioulas 2011 {published and unpublished data}
-
- NCT01319695. Corifollitropin alfa versus recombinant follicle stimulating hormone (FSH) in ovarian stimulation of women undergoing in vitro fertilisation. clinicaltrials.gov/show/NCT01319695 (accessed 8 March 2011).
-
- Sioulas V, Siristatidis C, Chrelias C, Alexiou E, Vrantza T, Kassanos D. Corifollitropin alfa vs. recombinant follicle stimulating hormone in ovarian stimulation of women undergoing in vitro fertilization: Preliminary results. Fertility and Sterility. 2011; Vol. 96 Suppl:S181 P‐252.
References to studies excluded from this review
Balen 2004 {published and unpublished data}
-
- Balen AH, Mulders AG, Fauser BC, Schoot BC, Renier MA, Devroey P, et al. Pharmacodynamics of a single low dose of long‐acting recombinant follicle‐stimulating hormone (FSH‐carboxy terminal peptide, corifollitropin alfa) in women with World Health Organization group II anovulatory infertility. Journal of Clinical Endocrinology and Metabolism 2004;89(12):6297‐304. - PubMed
Croxtall 2011 {published and unpublished data}
-
- Croxtall JD, McKeage K. Corifollitropin alfa: a review of its use in controlled ovarian stimulation for assisted reproduction. BioDrugs 2011;25(4):243‐54. - PubMed
de Lartigue 2011 {published and unpublished data}
-
- Lartigue J. Corifollitropin alfa: a new option to treat female infertility. Drugs of Today 2011;47(8):583‐90. - PubMed
Fatemi 2010 {published and unpublished data}
-
- Fatemi HM, Oberyé J, Popovic‐Todorovic B, Witjes H, Mannaerts B, Devroey P. Corifollitropin alfa in a long GnRH agonist protocol: proof of concept trial. Fertility and Sterility 2010;94(5):1922‐4. - PubMed
Leader 2013 {published and unpublished data}
Ledger 2009 {published and unpublished data}
-
- Ledger W, Zandvliet A, Mannaerts B. On behalf on the ENGAGE/ENSURE investigators: Two doses of corifollitropin alfa allow for similar exposure and ovarian response in patients weighing ≤60 kg and >60 kg. Molecular Human Reproduction 2009;24(Suppl 1):i114.
Loutradis 2010 {published and unpublished data}
-
- Loutradis D, Vlismas A, Drakakis P. Corifollitropin alfa: a novel long‐acting recombinant follicle‐stimulating hormone agonist for controlled ovarian stimulation. Women's Health 2010;6(5):655‐64. - PubMed
Mahmoud Youssef 2012 {published and unpublished data}
-
- Mahmoud Youssef MA, Wely M, Aboulfoutouh I, El‐Khyat W, Veen F, Al‐Inany H. Is there a place for corifollitropin alfa in IVF/ICSI cycles? A systematic review and meta‐analysis. Fertility and Sterility 2012;97(4):876‐85. - PubMed
Norman 2011 {published and unpublished data}
Prados 2011 {published and unpublished data}
-
- Prados N, Pellicer A, Fernandez‐Sanchez M. Corifollitropin alfa: A new recombinant FSH gonadotropin analogue. Expert Review of Obstetrics and Gynecology 2011;6(4):395‐402.
Rombauts 2012 {published and unpublished data}
-
- Rombauts L, Talmor A. Corifollitropin alfa for female infertility. Expert Opinion on Biological Therapy 2012;12(1):107‐12. - PubMed
Seyhan 2011 {published and unpublished data}
Talmor 2013 {published and unpublished data}
-
- Talmor A, McLachlan V, Osianlis T, Sorby KL, Lutjen P. Is longer better? The long and short of it ‐ a matched case control study of long and short acting follicle stimulating hormone. Fertility and Sterility 2013;100(Supplement 3):S17.
Additional references
Al‐Inany 2011
de Greef 2010
-
- Greef R, Zandvliet AS, Haan AFJ, Ijzerman‐Boon PCI, Marintcheva‐Petrova M, Mannaerts BMJL. Dose selection of corifollitropin alfa by modeling and simulation in controlled ovarian stimulation. Clinical Pharmacology and Therapeutics 2010;88(1):79‐87. - PubMed
Devroey 2009
-
- Devroey P, Boostanfar R, Koper NP, Mannaerts BMJL, IJzerman‐Boon PC, Fauser BCJM, the ENGAGE Investigators. A double‐blind, non‐inferiority RCT comparing corifollitropin alfa and recombinant FSH during the first seven days of ovarian stimulation using a GnRH antagonist protocol. Human Reproduction 2009;24(12):3063‐72. - PMC - PubMed
Duijkers 2002
-
- Duijkers IJ, Klipping C, Boerrigter PJ, Machielsen CS, Bie JJ, Voortman G. Single dose pharmacokinetics and effects on follicular growth and serum hormones of a long‐acting recombinant FSH preparation (FSH‐CTP) in healthy pituitary‐suppressed females. Human Reproduction 2002;17(8):1987‐93. - PubMed
Evers 2002
-
- Evers JLH. Female subfertility. Lancet 2002;360(9327):151‐9. - PubMed
Fauser 2009
-
- Fauser BC, Mannaerts BM, Devroey P, Leader A, Boime I, Baird DT. Advances in recombinant DNA technology: corifollitropin alfa, a hybrid molecule with sustained follicle‐stimulating activity and reduced injection frequency. Human Reproduction Update 2009;15(3):309‐21. - PubMed
Gnoth 2005
-
- Gnoth C, Godehardt E, Frank‐Herrmann P, Friol K, Tigges J, Freundl G. Definition and prevalence of subfertility and infertility. Human Reproduction 2005;20(5):1144‐7. - PubMed
Heineman 2007
-
- Heineman MJ, Evers JLH, Massuger LFAG, Steegers EAP. Obstetrie en Gynaecology. De voortplanting van de mens. Amsterdam: Elsevier Gezondheidszorg, 2007.
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kovacs 2011
-
- Kovacs G. How to Improve your ART Success Rates. An Evidence‐Based Review of Adjuncts to IVF. Cambridge: Cambridge University Press, 2011.
Maheshwari 2011
MDSG module 2008
-
- Farquhar C, Clarke J, Lethaby A, Thomas J, Proctor M, Barlow D, et al. Cochrane Menstrual Disorders and Subfertility Group, About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). Available at www.thecochranelibrary.com 2008, issue 4.
RevMan 2014 [Computer program]
-
- The Nordic Cochrane Centre, the Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, the Cochrane Collaboration, 2014.
Schröder 2004
-
- Schröder AK, Katalinic A, Diedrich K, Ludwig M. Cumulative pregnancy rates and drop‐out rates in a German IVF programme: 4102 cycles in 2130 patients. Reproductive Biomedicine Online 2004;8(5):600‐6. - PubMed
Tarlatzis 2007
-
- Tarlatzis BC, Kolibianakis EM. GnRH agonists vs antagonists. Best Practice & Research. Clinical Obstetrics and Gynaecology 2007;21(1):57‐65. - PubMed
Tur‐Kaspa
-
- Tur‐Kaspa I, Fauser B. The use of GnRH agonist in IVF protocols. IVF Worldwide website accessed 20 May 2015:http://www.ivf‐worldwide.com/survey/the‐use‐of‐gnrh‐agonist‐in‐ivf‐protocols/results‐th....
References to other published versions of this review
Pouwer 2012a
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

