Interventions for preventing the progression of autosomal dominant polycystic kidney disease
- PMID: 26171904
- PMCID: PMC8406618
- DOI: 10.1002/14651858.CD010294.pub2
Interventions for preventing the progression of autosomal dominant polycystic kidney disease
Update in
-
Interventions for preventing the progression of autosomal dominant polycystic kidney disease.Cochrane Database Syst Rev. 2024 Oct 2;10(10):CD010294. doi: 10.1002/14651858.CD010294.pub3. Cochrane Database Syst Rev. 2024. PMID: 39356039 Free PMC article.
Abstract
Background: Autosomal dominant polycystic kidney disease (ADPKD) is the most common inherited disorder causing kidney disease. Current clinical management of ADPKD focuses primarily on symptom control and reducing associated complications, particularly hypertension. In recent years, improved understanding of molecular and cellular mechanisms involved in kidney cyst growth and disease progression has resulted in new pharmaceutical agents to target disease pathogenesis to prevent progressive disease.
Objectives: We aimed to evaluate the effects of interventions for preventing ADPKD progression on kidney function, kidney endpoints, kidney structure, patient-centred endpoints (such as cardiovascular events, sudden death, all-cause mortality, hospitalisations, BP control, quality of life, and kidney pain), as well as the general and specific adverse effects related to their use.
Search methods: We searched the Cochrane Renal Group's Specialised Register to 6 June 2015 using relevant search terms.
Selection criteria: Randomised controlled trials (RCTs) comparing any interventions for preventing the progression of ADPKD with other interventions or placebo were considered for inclusion without language restriction.
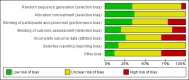
Data collection and analysis: Two authors independently assessed study risks of bias and extracted data. We summarised treatment effects on clinical outcomes, kidney function and structure and adverse events using random effects meta-analysis. We assessed heterogeneity in estimated treatment effects using the Cochran Q test and I(2) statistic. Summary treatment estimates were calculated as a mean difference (MD) or standardised mean difference (SMD) for continuous outcomes and a risk ratio (RR) for dichotomous outcomes together with their 95% confidence intervals.
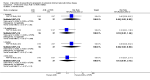
Main results: We included 30 studies (2039 participants) that investigated 11 pharmacological interventions (angiotensin-converting enzyme inhibitors (ACEi), angiotensin receptor blockers (ARBs), calcium channel blockers, beta blockers, vasopressin receptor 2 (V2R) antagonists, mammalian target of rapamycin (mTOR) inhibitors, somatostatin analogues, antiplatelet agents, eicosapentaenoic acids, statins and vitamin D compounds) in this review.ACEi significantly reduced diastolic blood pressure (9 studies, 278 participants: MD -4.96 mm Hg, 95% CI -8.88 to -1.04), but had uncertain effects on kidney volumes (MD -42.50 mL, 95% CI -115.68 to 30.67), GFR (MD -3.41 mL/min/1.73 m(2), 95% CI -15.83 to 9.01), and SCr (MD -0.02 mg/dL, 95% CI -0.14 to 0.09), in data largely restricted to children. ACEi did not show different effects on GFR (MD -8.19 mL/min/1.73 m(2), 95% CI -29.46 to 13.07) and albuminuria (SMD -0.19, 95% CI -1.77 to 1.39) when compared with beta-blockers, or SCr (MD 0.00 mg/dL, 95% CI -0.09 to 0.10) when compared with ARBs.Data for effects of V2R antagonists on kidney function and volumes compared to placebo were limited to narrative information within a single study while these agents increased thirst (1444 participants: RR 2.70, 95% CI 2.24 to 3.24) and dry mouth (1455 participants: RR 1.33, 95% CI 1.01 to 1.76).Compared with no treatment, mTOR inhibitors had uncertain effects on kidney function (2 studies, 115 participants: MD 4.45 mL/min/1.73 m(2), 95% CI -3.20 to 12.11) and kidney volume (MD -0.08 L, 95% CI -0.75 to 0.59) but in three studies (560 participants) caused angioedema (RR 13.39, 95% CI 2.56 to 70.00), oral ulceration (RR 6.77, 95% CI 4.42 to 10.38), infections (RR 1.14, 95% CI 1.04 to 1.25) and diarrhoea (RR 1.70, 95% CI 1.26 to 2.29).Somatostatin analogues (6 studies, 138 participants) slightly improved SCr (MD -0.43 mg/dL, 95% CI -0.86 to -0.01) and total kidney volume (MD -0.62 L, 95% CI -1.22 to -0.01) but had no definite effects on GFR (MD 9.50 mL/min, 95% CI -4.45 to 23.44) and caused diarrhoea (RR 3.72, 95% CI 1.43 to 9.68).Data for calcium channel blockers, eicosapentaenoic acids, statins, vitamin D compounds and antiplatelet agents were sparse and inconclusive.Random sequence generation was adequate in eight studies, and in almost half of the studies, blinding was not present or not specified. Most studies did not adequately report outcomes, which adversely affected our ability to assess this bias. The overall drop-out rate was over 10% in nine studies, and few were conducted using intention-to-treat analyses.
Authors' conclusions: Although several interventions are available for patients with ADPKD, at present there is little or no evidence that treatment improves patient outcomes in this population and is associated with frequent adverse effects. Additional large randomised studies focused on patient-centred outcomes are needed.
Conflict of interest statement
Davide Bolignano: none known
Suetonia C Palmer: none known
Marinella Ruospo: none known
Carmine Zoccali: none known
Jonathan C Craig: none known
Giovanni FM Strippoli: none known.
Figures










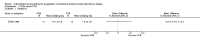










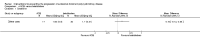
















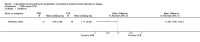




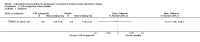




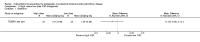











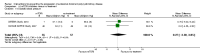


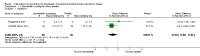






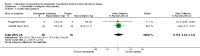

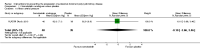






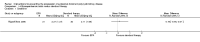







References
References to studies included in this review
AIPRI Study 1996 {published data only}
-
- Apperloo AJ, Rensma PL. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596‐7. [MEDLINE: ] - PubMed
-
- Curren CG. Effect of benazepril in chronic renal insufficiency. New England Journal of Medicine 1996;335(8):596‐7. [MEDLINE: ] - PubMed
-
- Hogan TJ, Elliott WJ, Seto AH, Bakris GL. Antihypertensive treatment with and without benazepril in patients with chronic renal insufficiency: a US economic evaluation. Pharmacoeconomics 2002;20(1):37‐47. [MEDLINE: ] - PubMed
-
- Locatelli F, Carbarns IR, Maschio G, Mann JF, Ponticelli C, Ritz E, et al. Long‐term progression of chronic renal insufficiency in the AIPRI Extension Study. The Angiotensin‐Converting‐Enzyme Inhibition in Progressive Renal Insufficiency Study Group. Kidney International ‐ Supplement 1997;63:S63‐6. [MEDLINE: ] - PubMed
-
- Locatelli F, Vecchio L, Andrulli S, Marai P, Tentori F. The role of underlying nephropathy in the progression of renal disease. Kidney International ‐ Supplement 2000;75:S49‐55. [MEDLINE: ] - PubMed
ALADIN Study 2013 {published data only}
-
- Caroli A, Perico N, Perna A, Antiga L, Brambilla P, Pisani A, et al. Effect of longacting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): a randomised, placebo‐controlled, multicentre trial. Lancet 2013;382(9903):1485‐95. [MEDLINE: ] - PubMed
Biao 1997 {published data only}
-
- Biao S. Clinical observation on adult polycystic kidney disease treated with calcitriol and 'qijudihuang mixt' [abstract no: P1057]. Nephrology 1997;3(Suppl 1):S338. [CENTRAL: CN‐00460393]
Cadnapaphornchai 2005 {published data only}
-
- Cadnapaphornchai MA, Fick‐Brosnahan GM, Duley I, Johnson AM, Strain JD, DeGroff CG, et al. Design and baseline characteristics of participants in the study of antihypertensive therapy in children and adolescents with autosomal dominant polycystic kidney disease (ADPKD). Contemporary Clinical Trials 2005;26(2):211‐22. [MEDLINE: ] - PubMed
Cadnapaphornchai 2005 borderline {published data only}
-
- Cadnapaphornchai MA, Fick‐Brosnahan GM, Duley I, Johnson AM, Strain JD, DeGroff CG, et al. Design and baseline characteristics of participants in the study of antihypertensive therapy in children and adolescents with autosomal dominant polycystic kidney disease (ADPKD). Contemporary Clinical Trials 2005;26(2):211‐22. [MEDLINE: ] - PubMed
Cadnapaphornchai 2005 normotensive {published data only}
-
- Cadnapaphornchai MA, Fick‐Brosnahan GM, Duley I, Johnson AM, Strain JD, DeGroff CG, et al. Design and baseline characteristics of participants in the study of antihypertensive therapy in children and adolescents with autosomal dominant polycystic kidney disease (ADPKD). Contemporary Clinical Trials 2005;26(2):211‐22. [MEDLINE: ] - PubMed
Ecder 1999 {published data only}
-
- Ecder T, Chapman AB, Brosnahan GM, Edelstein CL, Johnson AM, Schrier RW. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. American Journal of Kidney Diseases 2000;35(3):427‐32. [MEDLINE: ] - PubMed
-
- Ecder T, Edelstein CL, Chapman AB, Johnson AM, Tison L, Gill EA, et al. Reversal of left ventricular hypertrophy with angiotensin converting enzyme inhibition in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 1999;14(5):1113‐6. [MEDLINE: ] - PubMed
-
- Ecder T, McFann KK, Johnson AM, Chapman CB, Edelstein CL, Tison M, et al. Reversal of left ventricular hypertrophy in autosomal dominant polycystic kidney disease (ADPKD) patients with rigorous blood pressure (BP) control [abstract]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):534A. [CENTRAL: CN‐00550560]
-
- Schrier R, McFann K, Johnson A, Chapman A, Edelstein C, Brosnahan G, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal‐dominant polycystic kidney disease: results of a seven‐year prospective randomized study. Journal of the American Society of Nephrology 2002;13(7):1733‐9. [MEDLINE: ] - PubMed
ELATE Study 2011 {published data only}
-
- Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: Results from a randomized controlled trial in polycystic liver disease patients [abstract]. Journal of Hepatology 2013;58(Suppl 2):S557‐8. [EMBASE: 71055656] - PubMed
-
- Chrispijn M, Gevers TJ, Hol JC, Monshouwer R, Dekker HM, Drenth JP. Everolimus does not further reduce polycystic liver volume when added to long acting octreotide: results from a randomized controlled trial. Journal of Hepatology 2013;59(1):153‐9. [MEDLINE: ] - PubMed
Fassett 2010 {published data only}
-
- Fassett RG, Coombes JS, Packham D, Fairley KF, Kincaid‐Smith P. Effect of pravastatin on kidney function and urinary protein excretion in autosomal dominant polycystic kidney disease. Scandinavian Journal of Urology & Nephrology 2010;44(1):56‐61. [MEDLINE: ] - PubMed
-
- Kincaid‐Smith P, Vincent J, Fairley K. Randomised controlled trial of HMG CO A reductase inhibitor in polycystic renal disease in man [abstract no: A1733]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):375A. [CENTRAL: CN‐00446097]
Higashihara 2008 {published data only}
-
- Higashihara E, Nutahara K, Horie S, Muto S, Hosoya T, Hanaoka K, et al. The effect of eicosapentaenoic acid on renal function and volume in patients with ADPKD. Nephrology Dialysis Transplantation 2008;23(9):2847‐52. [MEDLINE: ] - PubMed
Hogan 2010 {published data only}
-
- Hogan M, Masyuk TV, Torres V, King BF, Kim BF, LaRusso NF. OctreotideLAR inhibits hepatorenal cystogenesis in the human polycystic liver diseases. Hepatology 2009;50(Suppl 4):328A. [EMBASE: 70073470]
LOCKCYST Study 2009 {published data only}
-
- Chrispijn M, Keimpema L, Nevens F, Vanslembrouck R, Oijen MG, Hoffmann AD, et al. Growth of liver volume stops after one year of lanreotide in patients with polycystic livers. Journal of Hepatology 2010;52(Suppl 1):S31. [EMBASE: 70130918]
-
- Chrispijn M, Nevens F, Gevers TJ, Vanslembrouck R, Oijen MG, Coudyzer W, et al. The long‐term outcome of patients with polycystic liver disease treated with lanreotide. Alimentary Pharmacology & Therapeutics 2012;35(2):266‐74. [MEDLINE: ] - PubMed
-
- Keimpema L, Nevens F, Vanslembrouck R, Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: A randomized, double‐blind, placebo‐controlled trial [abstract]. Hepatology 2009;50(Suppl 4):328A. - PubMed
-
- Keimpema L, Nevens F, Vanslembrouck R, Oijen MG, Hoffmann AL, Dekker HM, et al. Lanreotide reduces the volume of polycystic liver: a randomized, double‐blind, placebo‐controlled trial. Gastroenterology 2009;137(5):1661‐8. [MEDLINE: ] - PubMed
Melemadathil 2013 {published data only}
-
- Melemadathil S, Kamal M. Efficacy and safety of sirolimus in reducing cyst volume in patients with autosomal dominant polycystic kidney disease [abstract]. Nephrology Dialysis Transplantation 2013;28:i81‐2. [EMBASE: 71075211]
Mora 2013 {published data only}
-
- Mora FP, Codianni P, Liern M, Grammatico D, Vallejo G. Use of rapamycin to reduce the pathologic kidney volume growth in autosomal polycystic kidney disease [abstract]. Pediatric Nephrology 2013;28(8):1492. [EMBASE: 71127367]
Nakamura 2001d {published data only}
-
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al. Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80‐2. [MEDLINE: ] - PubMed
Nakamura 2001d hypertensive {published data only}
-
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al. Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80‐2. [MEDLINE: ] - PubMed
Nakamura 2001d normotensive {published data only}
-
- Nakamura T, Ushiyama C, Takahashi Y, Tanaka A, Shimada N, Ebihara I, et al. Effect of dilazep dihydrochloride on urinary albumin excretion in patients with autosomal dominant polycystic kidney disease. Nephron 2001;88(1):80‐2. [MEDLINE: ] - PubMed
Nakamura 2012a {published data only}
-
- Nakamura T, Sato E, Fujiwara N, Kawagoe Y, Yamada S, Ueda Y, et al. Changes in urinary albumin excretion, inflammatory and oxidative stress markers in ADPKD patients with hypertension. American Journal of the Medical Sciences 2012;343(1):46‐51. [MEDLINE: ] - PubMed
Nutahara 2005 {published data only}
-
- Nutahara K, Higashihara E, Horie S, Kamura K, Tsuchiya K, Mochizuki T, et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron 2005;99(1):c18‐23. [MEDLINE: ] - PubMed
RAPYD Study 2012 {published data only}
-
- Stallone G, Infante B, Bruno F, Bristogiannis C, Grandaliano G, Macarini L, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (ADPKD) study: a randomized, controlled study [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii46‐7. [EMBASE: 70765435] - PubMed
-
- Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD‐study): a randomized, controlled study. Nephrology Dialysis Transplantation 2012;27(9):3560‐7. [MEDLINE: ] - PubMed
RAPYD Study 2012 high {published data only}
-
- Stallone G, Infante B, Bruno F, Bristogiannis C, Grandaliano G, Macarini L, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (ADPKD) study: a randomized, controlled study [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii46‐7. [EMBASE: 70765435] - PubMed
-
- Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD‐study): a randomized, controlled study. Nephrology Dialysis Transplantation 2012;27(9):3560‐7. [MEDLINE: ] - PubMed
RAPYD Study 2012 low {published data only}
-
- Stallone G, Infante B, Bruno F, Bristogiannis C, Grandaliano G, Macarini L, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (ADPKD) study: a randomized, controlled study [abstract]. Nephrology Dialysis Transplantation 2012;27(Suppl 2):ii46‐7. [EMBASE: 70765435] - PubMed
-
- Stallone G, Infante B, Grandaliano G, Bristogiannis C, Macarini L, Mezzopane D, et al. Rapamycin for treatment of type I autosomal dominant polycystic kidney disease (RAPYD‐study): a randomized, controlled study. Nephrology Dialysis Transplantation 2012;27(9):3560‐7. [MEDLINE: ] - PubMed
Ruggenenti 2005 {published data only}
-
- Ruggenenti P, Remuzzi A, Ondei P, Fasolini G, Antiga L, Ene‐Iordache B, et al. Safety and efficacy of long‐acting somatostatin treatment in autosomal‐dominant polycystic kidney disease. Kidney International 2005;68(1):206‐16. [MEDLINE: ] - PubMed
SIRENA Study 2010 {published data only}
Soliman 2009 {published data only}
-
- Soliman A, Zamil S, Lotfy A, Ismail E. Sirolimus produced S‐shaped effect on adult polycystic kidneys after 2‐year treatment. Transplantation Proceedings 2012;44(10):2936–9. [MEDLINE: ] - PubMed
-
- Soliman AR, Ismail E. Sirolimus therapy for patients with adult polycystic kidney disease ‐ a pilot study [abstract no: TH‐PO053]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):123A. [CENTRAL: CN‐00716073] - PubMed
-
- Soliman AR, Ismail E, Zamil S, Lotfy A. Sirolimus therapy for patients with adult polycystic kidney disease: a pilot study. Transplantation Proceedings 2009;41(9):3639–41. [MEDLINE: ] - PubMed
SUISSE ADPKD Study 2007 {published data only}
-
- Braun M, Young J, Reiner CS, Poster D, Wuthrich RP, Serra AL. Ovarian toxicity from sirolimus. New England Journal of Medicine 2012;366(11):1062‐4. [MEDLINE: ] - PubMed
-
- Serra A, Poster D, Kistler AD, Krauer F, Raina F, Voneckardstein A, et al. Safety, tolerability and adherence of sirolimus in autosomal dominant polycystic kidney disease [abstract no: 2.5]. Swiss Medical Weekly 2008;138(Suppl 167):4S.
-
- Serra AL, Kistler AD, Poster D, Krauer F, Senn O, Raina S, et al. Safety and tolerability of sirolimus treatment in patients with autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2009;24(11):3334‐42. [MEDLINE: ] - PubMed
Temmerman 2012 {published data only}
-
- Temmerman F, Vanslembrouck R, Coudyzer W, Bammens B, Laleman W, Cassiman D, et al. The reduction in liver volume in polycystic liver disease with lanreotide is dose dependent and is most pronounced in patients with the highest liver volume [abstract]. Journal of Hepatology 2012;56:S547. [EMBASE: 70749518]
TEMPO 248 & 249 2005 {published data only}
-
- Chapman AB, Torres VE, Grantham JJ, Shoaf SS, Ouyang JJ, Czerwiec FS. A phase IIB pilot study of the safety and efficacy of tolvaptan, a vasopressin V2 receptor antagonist (V2RA), in patients with ADPKD [abstract no: F‐FC139]. Journal of the American Society of Nephrology 2005;16:68A. [CENTRAL: CN‐00653783]
-
- Grantham JJ, Chapman AB, Torres VE, Ouyang JJ, Shoaf SE, Czerwiec FS. Acute and chronic osmostasis after vasopressin V2 receptor inhibition with tolvaptan in ADPKD [abstract no: F‐PO106]. Journal of the American Society of Nephrology 2005;16(October):361A. [CENTRAL: CN‐00653784]
-
- Torres VE, Wang X, Ward CJ, Grantham JJ, Chapman AB, Ouyang JJ, et al. Urine aquaporin 2 and cyclic AMP responses to tolvaptan administration in autosomal dominant polycystic kidney disease [abstract no: F‐PO108]. Journal of the American Society of Nephrology 2005;16(October):361A. [CENTRAL: CN‐00653785]
TEMPO 250 2011 {published data only}
-
- Torres VE, Grantham JJ, Chapman AB, Watnick T, Kedzierski K, Ouyang JJ, et al. Phase 2 open‐label study to determine safety, tolerability and efficacy of split‐dose tolvaptan in ADPKD [abstract no: SA‐PO077]. Journal of the American Society of Nephrology 2007;18:361A‐2A. [CENTRAL: CN‐00653786]
TEMPO 3‐4 Study 2011 {published data only}
-
- Devuyst O, Chapman AB, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, et al. Urine osmolality and outcome in ADPKD: Results from the TEMPO 3:4 trial [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii6. [EMBASE: 71491481]
-
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Higashihara E, Perrone RD, et al. Tolvaptan‐treatment of ADPKD confers persistent EGFR improvement: Results from the TEMPO 4:4 extension trial [abstract]. Nephrology Dialysis Transplantation 2014;29(Suppl 3):iii6. [EMBASE: 71491483]
-
- Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, et al. Rationale and design of the TEMPO (Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes) 3‐4 Study. American Journal of Kidney Diseases 2011;57(5):692‐9. [MEDLINE: ] - PubMed
Ulusoy 2010 {published data only}
-
- Ulusoy S, Ozkan G, Orem C, Kaynar K, Kosucu P, Kiris A. A comparison of the effects of ramipril and losartan on blood pressure control and left ventricle hypertrophy in patients with autosomal dominant polycystic kidney disease. Renal Failure 2010;32(8):913‐7. [MEDLINE: ] - PubMed
van Dijk 2001 {published data only}
-
- Dijk MA, Kamper AM, Veen S, Souverijn JH, Blauw GJ. Effect of simvastatin on renal function in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2001;16(11):2152‐7. [MEDLINE: ] - PubMed
van Dijk 2003 {published data only}
-
- Dijk MA, Breuning MH, Duiser R, Es LA, Westendorp RG. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2003;18(11):2314‐20. [MEDLINE: ] - PubMed
Walz 2010 {published data only}
-
- Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, et al. Everolimus in patients with autosomal dominant polycystic kidney disease. New England Journal of Medicine 2010;363(9):830‐40. [MEDLINE: ] - PubMed
Watson 1999 {published data only}
-
- Watson ML, Macnicol AM, Borg‐Costanzi J, Vareesanghip K, Chauveau D, Cohen G, et al. A long‐term comparison of the effects of renal function of BP control with either atenolol (A) or enalapril (E) in polycystic kidney disease (PKD) [abstract]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):428A.
Zeltner 2008 {published data only}
-
- Mueller H, Schmieder RE, Zeltner R, Poliak R, Graf S, Schulze BD. Determinants for the treatment of hypertensive patients with autosomal dominant polycystic kidney disease (ADPKD): choice of drug versus blood pressure (BP) control [abstract]. Journal of the American Society of Nephrology 2003;14(Nov):109A. [CENTRAL: CN‐00653777]
-
- Zeltner R, Poliak R, Stiasny B, Schmieder RE, Schulze BD. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrology Dialysis Transplantation 2008;23(2):573‐9. [MEDLINE: ] - PubMed
References to studies excluded from this review
Doulton 2006 {published data only}
-
- Doulton TW, Saggar‐Malik AK, He FJ, Carney C, Markandu ND, Sagnella GA, et al. The effect of sodium and angiotensin‐converting enzyme inhibition on the classic circulating renin‐angiotensin system in autosomal‐dominant polycystic kidney disease patients. Journal of Hypertension 2006;24(5):939‐45. [MEDLINE: ] - PubMed
ISRCTN57653760 {published data only}
-
- O'Shaugnessy K. A rotation study through the main therapeutic classes of antihypertensive in patients with polycystic kidney disease and hypertension. controlled‐trials.com/ISRCTN57653760 (accessed 1 June 2015).
Kanno 1996 {published data only}
-
- Kanno Y, Suzuki H, Okada H, Takenaka T, Saruta T. Calcium channel blockers versus ACE inhibitors as antihypertensives in polycystic kidney disease. Qjm 1996;89(1):65‐70. [MEDLINE: ] - PubMed
-
- Suzuki H, Kanno Y, Okada H, Konishi K, Nakazato Y, Okamiya Y, et al. Renal protective effects of calcium channel blocker on hypertensive patients with autosomal dominant polycystic kidney disease [abstract]. Journal of the American Society of Nephrology 1994;5(3):568.
Nakamura 2005a {published data only}
-
- Nakamura T, Sugaya T, Kawagoe Y, Ueda Y, Osada S, Koide H. Candesartan reduces urinary fatty acid‐binding protein excretion in patients with autosomal dominant polycystic kidney disease. American Journal of the Medical Sciences 2005;330(4):161‐5. [MEDLINE: ] - PubMed
Sharma 2004 {published data only}
-
- Sharma RK, Kohli R, Rathore D, Gupta A, Gupta RK. Magnetic resonance based studies as a marker of disease progression in autosomal‐dominant polycystic kidney disease and the effect of simvastatin on disease progression [abstract]. Indian Journal of Nephrology 2004;14:126.
References to studies awaiting assessment
Braun 2014 {published data only}
Cadnapaphornchai 2011 {published data only}
-
- Cadnapaphornchai MA, George DM, McFann K, Wang W, Gitomer B, Strain JD, et al. Effect of pravastatin on total kidney volume, left ventricular mass index, and microalbuminuria in pediatric autosomal dominant polycystic kidney disease. Clinical Journal of the American Society of Nephrology: CJASN 2014;9(5):889‐96. [MEDLINE: ] - PMC - PubMed
HALT‐PKD Study 2008 {published data only}
-
- Chapman AB. Approaches to testing new treatments in autosomal dominant polycystic kidney disease: insights from the CRISP and HALT‐PKD studies. Clinical Journal of The American Society of Nephrology: CJASN 2008;3(4):1197‐204. [MEDLINE: ] - PubMed
-
- Miskulin D, Chapman A, Steinman T, Schrier R, Torres V, Perrone R, et al. Impact of autosomal dominant polycystic kidney disease on quality of life: results from the HALT‐PKD study [abstract no: TH‐PO052]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):123A. [CENTRAL: CN‐00756923]
NCT01233869 {published data only}
-
- NCT01233869. A phase 2, multicenter, randomized, double‐blind, placebo‐controlled study of the safety, clinical activity and pharmacokinetics of bosutinib (PF‐05208763) versus placebo in subjects with autosomal dominant polycystic kidney disease (ADPKD). www.clinicaltrials.gov/ct2/show/NCT01233869 2014. [MEDLINE: ]
References to ongoing studies
DIPAK 1 Study 2014 {published data only}
-
- Meijer E, Drenth JP, d'Agnolo H, Casteleijn NF, Fijter JW, Gevers TJ, et al. Rationale and design of the DIPAK 1 Study: a randomized controlled clinical trial assessing the efficacy of lanreotide to halt disease progression in autosomal dominant polycystic kidney disease. American Journal of Kidney Diseases 2014;63(3):446‐55. [MEDLINE: ] - PMC - PubMed
NCT00345137 {published data only}
-
- NCT00345137. Phase 1 study of systemic effects of Ng‐monomethyl‐L‐arginine on renal hemodynamics in patients with polycystic kidney disease and chronic glomerulonephritis. www.clinicaltrials.gov/ct2/show/NCT00345137 (accessed 1 June 2015).
NCT01932450 {published data only}
-
- NCT01932450. A randomized, open‐label study investigating the effect of bilateral renal artery sympathetic denervation by catheter‐based radiofrequency ablation on blood pressure and disease progression in autosomal dominant polycystic kidney disease. www.clinicaltrials.gov/ct2/show/NCT01932450 2013. [CENTRAL: CN‐00874871]
Additional references
Chang 2012
-
- Chang MY, Ong AC. Mechanism‐based therapeutics for autosomal dominant polycystic kidney disease: recent progress and future prospects. Nephron Clinical Practice 2012;120(1):c25‐34. [MEDLINE: ] - PubMed
Ecder 2013
-
- Ecder T. Cardiovascular complications in autosomal dominant polycystic kidney disease. Current Hypertension Reviews 2013;9(1):2‐11. [MEDLINE: ] - PubMed
ERA‐EDTA 2011
-
- ERA‐EDTA Registry. ERA‐EDTA Registry Annual Report 2011. Academic Medical Center, Department of Medical Informatics, Amsterdam, The Netherlands, 2011. www.era‐edta‐reg.org/files/annualreports/pdf/AnnRep2011.pdf (accessed 1 June 2015).
Gattone 2003
-
- Gattone VH 2nd, Wang X, Harris PC, Torres VE. Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nature Medicine 2003;9(10):1323–6. [MEDLINE: ] - PubMed
Gile 1995
-
- Gile RD, Cowley BD Jr, Gattone VH 2nd, O'Donnell MP, Swan SK, Grantham JJ. Effect of lovastatin on the development of polycystic kidney disease in the Han:SPRD rat. American Journal of Kidney Diseases 1995;26(3):501‐7. [MEDLINE: ] - PubMed
Grantham 2006
-
- Grantham JJ, Torres VE, Chapman AB, Guay‐Woodford LM, Bae KT, King BF Jr, et al. Volume progression in polycystic kidney disease. New England Journal of Medicine 2006;354(20):2122–30. [MEDLINE: ] - PubMed
Grantham 2008
Grantham 2011
-
- Grantham JJ, Bennett WM, Perrone RD. mTOR inhibitors and autosomal dominant polycystic kidney disease. New England Journal of Medicine 2011;364(3):286‐7. [MEDLINE: ] - PubMed
Hanaoka 2000
-
- Hanaoka K, Guggino W. cAMP regulates cell proliferation and cyst formation in autosomal polycystic kidney disease cells. Journal of the American Society of Nephrology 2000;11(7):1179‐87. [MEDLINE: ] - PubMed
Harris 2009
Helai 2012
-
- Helal I, Reed B, Schrier RW. Emergent early markers of renal progression in autosomal‐dominant polycystic kidney disease patients: implications for prevention and treatment. American Journal of Nephrology 2012;36(2):162‐7. [MEDLINE: ] - PubMed
Higgins 2003
Higgins 2011
-
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
Masyuk 2007
-
- Masyuk TV, Masyuk AI, Torres VE, Harris PC, Larusso NF. Octreotide inhibits hepatic cystogenesis in a rodent model of polycystic liver disease by reducing cholangiocyte adenosine 3’,5’‐cyclic monophosphate. Gastroenterology 2007;132(3):1104‐16. [MEDLINE: ] - PubMed
Nagao 2006
-
- Nagao S, Nishii K, Katsuyama M, Kurahashi H, Marunouchi T, Takahashi H, et al. Increased water intake decreases progression of polycystic kidney disease in the PCK rat. Journal of the American Society of Nephrology 2006;17(8):2220‐7. [MEDLINE: ] - PubMed
Ogborn 2000
-
- Ogborn MR, Nitschmann E, Weiler HA, Bankovic‐Calic N. Modification of polycystic kidney disease and fatty acid status by soy protein diet. Kidney International 2000;57(1):159‐66. [MEDLINE: ] - PubMed
Qian 2008
Rule 2006
-
- Rule AD, Torres VE, Chapman AB, Grantham JJ, Guay‐Woodford LM, Bae KT, et al. Comparison of methods for determining renal function decline in early autosomal dominant polycystic kidney disease: the consortium of radiologic imaging studies of polycystic kidney disease cohort. Journal of the American Society of Nephrology 2006;17(3):854‐62. [MEDLINE: ] - PubMed
Schrier 2009
-
- Schrier RW. Renal volume, renin‐angiotensin‐aldosterone system, hypertension, and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. Journal of the American Society of Nephrology 2009;20(9):1888‐93. [MEDLINE: ] - PubMed
Torres 2007
-
- Torres VE, Harris PC, Pirson Y. Autosomal dominant polycystic kidney disease. Lancet 2007;369(9569):1287‐301. [MEDLINE: ] - PubMed
Torres 2009
Torres 2012
USRDS 2008
-
- Table A.1.7 Incident counts of reported ESRD: all patients by age, gender, race, ethnicity, & primary diagnosis. IN: US Renal Data Services. Table A.1, Incident counts of reported ESRD: all patients. www.usrds.org/2008/ref/A_incidence_08.pdf (accessed 1 June 2015).
Wahl 2006
-
- Wahl PR, Serra AL, Hir M, Molle KD, Hall MN, Wüthrich RP. Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrology Dialysis Transplantation 2006;21(3):598‐604. [MEDLINE: ] - PubMed
Wu 2007
-
- Wu M, Wahl PR, Hir M, Wackerle‐Men Y, Wüthrich RP, Serra AL. Everolimus retards cyst growth and preserves kidney function in a rodent model for polycystic kidney disease. Kidney & Blood Pressure Research 2007;30(4):253‐9. [MEDLINE: ] - PubMed
Wüthrich 2009
-
- Wüthrich RP, Serra AL, Kistler AD. Autosomal dominant polycystic kidney disease: new treatment options and how to test their efficacy. Kidney & Blood Pressure Research 2009;32(5):380‐7. [MEDLINE: ] - PubMed
References to other published versions of this review
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

