Effect of pH on Cleavage of Glycogen by Vaginal Enzymes
- PMID: 26171967
- PMCID: PMC4501710
- DOI: 10.1371/journal.pone.0132646
Effect of pH on Cleavage of Glycogen by Vaginal Enzymes
Abstract
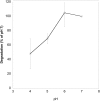
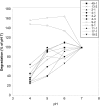
Glycogen expressed by the lower genital tract epithelium is believed to support Lactobacillus growth in vivo, although most genital isolates of Lactobacillus are not able to use glycogen as an energy source in vitro. We recently reported that α-amylase is present in the genital fluid of women and that it breaks down glycogen into small carbohydrates that support growth of lactobacilli. Since the pH of the lower genital tract can be very low, we determined how low pH affects glycogen processing by α-amylase. α-amylase in saliva degraded glycogen similarly at pH 6 and 7, but activity was reduced by 52% at pH 4. The glycogen degrading activity in nine genital samples from seven women showed a similar profile with an average reduction of more than 50% at pH 4. However, two samples collected from one woman at different times had a strikingly different pH profile with increased glycogen degradation at pH 4, 5 and 6 compared to pH 7. This second pH profile did not correlate with levels of human α-acid glucosidase or human intestinal maltase glucoamylase. High-performance anion-exchange chromatography showed that mostly maltose was produced from glycogen by samples with the second pH profile in contrast to genital α-amylase that yielded maltose, maltotriose and maltotetraose. These studies show that at low pH, α-amylase activity is reduced to low but detectable levels, which we speculate helps maintain Lactobacillus growth at a limited but sustained rate. Additionally, some women have a genital enzyme distinct from α-amylase with higher activity at low pH. Further studies are needed to determine the identity and distribution of this second enzyme, and whether its presence influences the makeup of genital microbiota.
Conflict of interest statement
Figures



Similar articles
-
Human α-amylase present in lower-genital-tract mucosal fluid processes glycogen to support vaginal colonization by Lactobacillus.J Infect Dis. 2014 Oct 1;210(7):1019-28. doi: 10.1093/infdis/jiu231. Epub 2014 Apr 15. J Infect Dis. 2014. PMID: 24737800 Free PMC article.
-
Amylases in the Human Vagina.mSphere. 2020 Dec 9;5(6):e00943-20. doi: 10.1128/mSphere.00943-20. mSphere. 2020. PMID: 33298571 Free PMC article.
-
Glycogen-Degrading Activities of Catalytic Domains of α-Amylase and α-Amylase-Pullulanase Enzymes Conserved in Gardnerella spp. from the Vaginal Microbiome.J Bacteriol. 2023 Feb 22;205(2):e0039322. doi: 10.1128/jb.00393-22. Epub 2023 Feb 6. J Bacteriol. 2023. PMID: 36744900 Free PMC article.
-
Intrinsic and extrinsic carbohydrates in the vagina: A short review on vaginal glycogen.Int J Biol Macromol. 2018 Jun;112:203-206. doi: 10.1016/j.ijbiomac.2018.01.166. Epub 2018 Jan 31. Int J Biol Macromol. 2018. PMID: 29391223 Review.
-
Unraveling the Dynamics of the Human Vaginal Microbiome.Yale J Biol Med. 2016 Sep 30;89(3):331-337. eCollection 2016 Sep. Yale J Biol Med. 2016. PMID: 27698617 Free PMC article. Review.
Cited by
-
Glycogen Levels in Undiluted Genital Fluid and Their Relationship to Vaginal pH, Estrogen, and Progesterone.PLoS One. 2016 Apr 19;11(4):e0153553. doi: 10.1371/journal.pone.0153553. eCollection 2016. PLoS One. 2016. PMID: 27093050 Free PMC article.
-
Characterization of a novel type of glycogen-degrading amylopullulanase from Lactobacillus crispatus.Appl Microbiol Biotechnol. 2022 Jun;106(11):4053-4064. doi: 10.1007/s00253-022-11975-2. Epub 2022 May 25. Appl Microbiol Biotechnol. 2022. PMID: 35612627
-
Dual probiotic and antibiotic therapy targeting bacterial vaginosis: an integrated experimental/computational modeling perspective.Biomed Eng Adv. 2025 Jun;9:100163. doi: 10.1016/j.bea.2025.100163. Epub 2025 Apr 2. Biomed Eng Adv. 2025. PMID: 40529156
-
Comparative study of vulva and abdominal skin microbiota of healthy females with high and average BMI.BMC Microbiol. 2019 Jan 17;19(1):16. doi: 10.1186/s12866-019-1391-0. BMC Microbiol. 2019. PMID: 30654751 Free PMC article.
-
Rationale and Safety Assessment of a Novel Intravaginal Drug-Delivery System with Sustained DL-Lactic Acid Release, Intended for Long-Term Protection of the Vaginal Microbiome.PLoS One. 2016 Apr 19;11(4):e0153441. doi: 10.1371/journal.pone.0153441. eCollection 2016. PLoS One. 2016. PMID: 27093291 Free PMC article. Clinical Trial.
References
-
- Amsel R, Totten PA, Spiegel CA, Chen KC, Eschenbach D, Holmes KK (1983) Nonspecific vaginitis. Diagnostic criteria and microbial and epidemiologic associations. Am J Med 74: 14–22. - PubMed
-
- Hillier SL (1998) The vaginal microbial ecosystem and resistance to HIV. AIDS Res Hum Retroviruses 14 Suppl 1: S17–21. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

