A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior
- PMID: 26172209
- PMCID: PMC4737929
- DOI: 10.1038/ismej.2015.119
A novel intracellular mutualistic bacterium in the invasive ant Cardiocondyla obscurior
Abstract
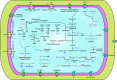
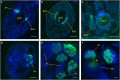
The evolution of eukaryotic organisms is often strongly influenced by microbial symbionts that confer novel traits to their hosts. Here we describe the intracellular Enterobacteriaceae symbiont of the invasive ant Cardiocondyla obscurior, 'Candidatus Westeberhardia cardiocondylae'. Upon metamorphosis, Westeberhardia is found in gut-associated bacteriomes that deteriorate following eclosion. Only queens maintain Westeberhardia in the ovarian nurse cells from where the symbionts are transmitted to late-stage oocytes during nurse cell depletion. Functional analyses of the streamlined genome of Westeberhardia (533 kb, 23.41% GC content) indicate that neither vitamins nor essential amino acids are provided for the host. However, the genome encodes for an almost complete shikimate pathway leading to 4-hydroxyphenylpyruvate, which could be converted into tyrosine by the host. Taken together with increasing titers of Westeberhardia during pupal stage, this suggests a contribution of Westeberhardia to cuticle formation. Despite a widespread occurrence of Westeberhardia across host populations, one ant lineage was found to be naturally symbiont-free, pointing to the loss of an otherwise prevalent endosymbiont. This study yields insights into a novel intracellular mutualist that could play a role in the invasive success of C. obscurior.
Figures


Similar articles
-
Deep Sequencing Uncovers Caste-Associated Diversity of Symbionts in the Social Ant Camponotus japonicus.mBio. 2020 Apr 21;11(2):e00408-20. doi: 10.1128/mBio.00408-20. mBio. 2020. PMID: 32317320 Free PMC article.
-
Evolution and Diversity of Inherited Spiroplasma Symbionts in Myrmica Ants.Appl Environ Microbiol. 2018 Jan 31;84(4):e02299-17. doi: 10.1128/AEM.02299-17. Print 2018 Feb 15. Appl Environ Microbiol. 2018. PMID: 29196290 Free PMC article.
-
Gene expression analysis of the endosymbiont-bearing midgut tissue during ontogeny of the carpenter ant Camponotus floridanus.J Insect Physiol. 2013 Jun;59(6):611-23. doi: 10.1016/j.jinsphys.2013.03.011. Epub 2013 Apr 6. J Insect Physiol. 2013. PMID: 23570961
-
Studying the Complex Communities of Ants and Their Symbionts Using Ecological Network Analysis.Annu Rev Entomol. 2016;61:353-71. doi: 10.1146/annurev-ento-010715-023719. Annu Rev Entomol. 2016. PMID: 26982442 Review.
-
Dissecting genome reduction and trait loss in insect endosymbionts.Ann N Y Acad Sci. 2017 Feb;1389(1):52-75. doi: 10.1111/nyas.13222. Epub 2016 Oct 10. Ann N Y Acad Sci. 2017. PMID: 27723934 Review.
Cited by
-
Links between metamorphosis and symbiosis in holometabolous insects.Philos Trans R Soc Lond B Biol Sci. 2019 Oct 14;374(1783):20190068. doi: 10.1098/rstb.2019.0068. Epub 2019 Aug 26. Philos Trans R Soc Lond B Biol Sci. 2019. PMID: 31438811 Free PMC article. Review.
-
To the Land and Beyond: Crab Microbiomes as a Paradigm for the Evolution of Terrestrialization.Front Microbiol. 2020 Oct 7;11:575372. doi: 10.3389/fmicb.2020.575372. eCollection 2020. Front Microbiol. 2020. PMID: 33117320 Free PMC article. Review.
-
A highly divergent Wolbachia with a tiny genome in an insect-parasitic tylenchid nematode.Proc Biol Sci. 2022 Sep 28;289(1983):20221518. doi: 10.1098/rspb.2022.1518. Epub 2022 Sep 28. Proc Biol Sci. 2022. PMID: 36168763 Free PMC article.
-
Physiological and evolutionary contexts of a new symbiotic species from the nitrogen-recycling gut community of turtle ants.ISME J. 2023 Oct;17(10):1751-1764. doi: 10.1038/s41396-023-01490-1. Epub 2023 Aug 9. ISME J. 2023. PMID: 37558860 Free PMC article.
-
Evidence of phylosymbiosis in Formica ants.Front Microbiol. 2023 May 5;14:1044286. doi: 10.3389/fmicb.2023.1044286. eCollection 2023. Front Microbiol. 2023. PMID: 37213490 Free PMC article.
References
-
- Akman L, Yamashita A, Watanabe H, Oshima K, Shiba T, Hattori M et al. (2002). Genome sequence of the endocellular obligate symbiont of tsetse flies Wigglesworthia glossinidia. Nat Genet 32: 402–407. - PubMed
-
- Alasaad S, Rossi L, Maione S, Sartore S, Soriguer RC, Pérez JM et al. (2008). HotSHOT Plus ThermalSHOCK, a new and efficient technique for preparation of PCR-quality mite genomic DNA. Parasitol Res 103: 1455–1457. - PubMed
-
- Andersen SO. (2012) Cuticular sclerotization and tanning. In: Gilbert LI (ed) Insect Molecular Biology and Biochemistry. Academic Press: Waltham, MA, USA, pp 167–192.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

