Population Pharmacokinetic Model of Sublingual Buprenorphine in Neonatal Abstinence Syndrome
- PMID: 26172282
- PMCID: PMC5119858
- DOI: 10.1002/phar.1610
Population Pharmacokinetic Model of Sublingual Buprenorphine in Neonatal Abstinence Syndrome
Abstract
Objective: Neonatal abstinence syndrome (NAS)--a clinical entity of infants from in utero exposure to psychoactive xenobiotic and buprenorphine--has been successfully used to treat NAS. However, nothing is known about the pharmacokinetics (PK) of buprenorphine in neonates with NAS. To our knowledge, this is the first study to investigate the population pharmacokinetic of sublingual buprenorphine in neonates with NAS.
Design: A retrospective population PK analysis of: (1) neonates with NAS treated with sublingual buprenorphine in randomized, double blinded clinical study and (2) data from healthy adults from a previously published pharmacokinetic study.
Setting: Neonatal intensive care unit and general clinical research unit.
Patients: Twenty-four neonates with NAS and five healthy adults.
Interventions: All participants received sublingual buprenorphine per study protocol.
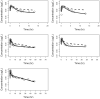
Measurements and main results: A total of 303 PK data from 29 neonates and adults were used for model development. A population pharmacokinetic analysis was conducted using a first order conditional estimation with interaction in the NONMEM software program. A two-compartment linear PK model with first-order absorption process best described the pharmacokinetics of sublingual buprenorphine in neonates. The apparent clearance (CL) of buprenorphine was linearly related to body weight and matured with increasing age via two distinct saturated pathways. A typical neonate with NAS (body weight, 2.9 kg; postnatal age; 5.4 days) had a CL of 3.5 L/kg/hour and elimination half-life of 11 hours. Phenobarbital did not affect the clearance of buprenorphine compared to neonates of similar age and weight.
Conclusions: This is the first study to investigate the population PK of sublingual buprenorphine in neonatal NAS. To our knowledge, this is also the first report to describe the age-dependent changes of buprenorphine PK in this patient population. No buprenorphine dose adjustment is needed for neonates with NAS treated with buprenorphine and concurrent phenobarbital.
Keywords: Neonatal Abstinence Syndrome; buprenorphine; pharmacokinetics.
© 2015 Pharmacotherapy Publications, Inc.
Figures



Similar articles
-
The Pharmacokinetics and Pharmacodynamics of Buprenorphine in Neonatal Abstinence Syndrome.Clin Pharmacol Ther. 2018 Jun;103(6):1029-1037. doi: 10.1002/cpt.1064. Epub 2018 Apr 28. Clin Pharmacol Ther. 2018. PMID: 29516490 Free PMC article. Clinical Trial.
-
Sublingual buprenorphine for treatment of neonatal abstinence syndrome: a randomized trial.Pediatrics. 2008 Sep;122(3):e601-7. doi: 10.1542/peds.2008-0571. Epub 2008 Aug 11. Pediatrics. 2008. PMID: 18694901 Free PMC article. Clinical Trial.
-
Buprenorphine versus methadone in the treatment of pregnant opioid-dependent patients: effects on the neonatal abstinence syndrome.Drug Alcohol Depend. 2005 Jul;79(1):1-10. doi: 10.1016/j.drugalcdep.2004.11.013. Drug Alcohol Depend. 2005. PMID: 15943939 Clinical Trial.
-
Breastfeeding promotion for management of neonatal abstinence syndrome.J Obstet Gynecol Neonatal Nurs. 2013 Sep-Oct;42(5):517-26. doi: 10.1111/1552-6909.12242. Epub 2013 Sep 4. J Obstet Gynecol Neonatal Nurs. 2013. PMID: 24020477 Review.
-
Buprenorphine in Neonatal Abstinence Syndrome.Clin Pharmacol Ther. 2018 Jan;103(1):112-119. doi: 10.1002/cpt.930. Epub 2017 Nov 28. Clin Pharmacol Ther. 2018. PMID: 29105752 Free PMC article. Review.
Cited by
-
Clinical pharmacology and dosing regimen optimization of neonatal opioid withdrawal syndrome treatments.Clin Transl Sci. 2021 Jul;14(4):1231-1249. doi: 10.1111/cts.12994. Epub 2021 May 1. Clin Transl Sci. 2021. PMID: 33650314 Free PMC article. Review.
-
Physiologic Indirect Response Modeling to Describe Buprenorphine Pharmacodynamics in Newborns Treated for Neonatal Opioid Withdrawal Syndrome.Clin Pharmacokinet. 2021 Feb;60(2):249-259. doi: 10.1007/s40262-020-00939-2. Clin Pharmacokinet. 2021. PMID: 32939690 Free PMC article.
-
The Pharmacokinetics and Pharmacodynamics of Buprenorphine in Neonatal Abstinence Syndrome.Clin Pharmacol Ther. 2018 Jun;103(6):1029-1037. doi: 10.1002/cpt.1064. Epub 2018 Apr 28. Clin Pharmacol Ther. 2018. PMID: 29516490 Free PMC article. Clinical Trial.
-
Neonatal abstinence syndrome: Pharmacologic strategies for the mother and infant.Semin Perinatol. 2016 Apr;40(3):203-12. doi: 10.1053/j.semperi.2015.12.007. Epub 2016 Jan 12. Semin Perinatol. 2016. PMID: 26791055 Free PMC article. Review.
-
Treating Chronic Pain: An Overview of Clinical Studies Centered on the Buprenorphine Option.Drugs. 2018 Aug;78(12):1211-1228. doi: 10.1007/s40265-018-0953-z. Drugs. 2018. PMID: 30051169 Free PMC article. Review.
References
-
- Hudak ML, Tan RC. Neonatal drug withdrawal. Pediatrics. 2012;129:e540–60. - PubMed
-
- Patrick SW, Schumacher RE, Benneyworth BD, Krans EE, Mc-Allister JM, Davis MM. Neonatal abstinence syndrome and associated health care expenditures: United States, 2000-2009. JAMA. 2012;307:1934–40. - PubMed
-
- Osborn DA, Jeffery HE, Cole MJ. Opiate treatment for opiate withdrawal in newborn infants. Cochrane Database Syst Rev. 2010;(10):CD002059. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

