Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure
- PMID: 26172304
- PMCID: PMC4695178
- DOI: 10.18632/oncotarget.4680
Exposure to ALS-FTD-CSF generates TDP-43 aggregates in glioblastoma cells through exosomes and TNTs-like structure
Abstract
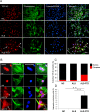
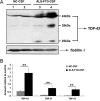
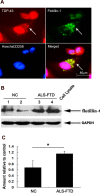
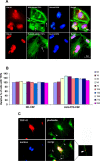
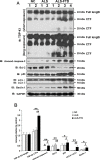
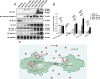
Amyotrophic lateral sclerosis (ALS) and frontotemporal dementia (FTD) represent a continuum of devastating neurodegenerative diseases, characterized by transactive response DNA-binding protein of 43 kDa (TDP-43) aggregates accumulation throughout the nervous system. Despite rapidly emerging evidence suggesting the hypothesis of 'prion-like propagation' of TDP-43 positive inclusion in the regional spread of ALS symptoms, whether and how TDP-43 aggregates spread between cells is not clear. Herein, we established a cerebrospinal fluid (CSF)-cultured cell model to dissect mechanisms governing TDP-43 aggregates formation and propagation. Remarkably, intracellular TDP-43 mislocalization and aggregates were induced in the human glioma U251 cells following exposure to ALS-FTD-CSF but not ALS-CSF and normal control (NC) -CSF for 21 days. The exosomes derived from ALS-FTD-CSF were enriched in TDP-43 C-terminal fragments (CTFs). Incubation of ALS-FTD-CSF induced the increase of mislocated TDP-43 positive exosomes in U251 cells. We further demonstrated that exposure to ALS-FTD-CSF induced the generations of tunneling nanotubes (TNTs)-like structure and exosomes at different stages, which mediated the propagation of TDP-43 aggregates in the cultured U251 cells. Moreover, immunoblotting analyses revealed that abnormal activations of apoptosis and autophagy were induced in U251 cells, following incubation of ALS-CSF and ALS-FTD-CSF. Taken together, our data provide direct evidence that ALS-FTD-CSF has prion-like transmissible properties. TNTs-like structure and exosomes supply the routes for the transfer of TDP-43 aggregates, and selective inhibition of their over-generations may interrupt the progression of TDP-43 proteinopathy.
Keywords: ALS; FTD; TDP-43; exosomes; tunneling nanotubes.
Conflict of interest statement
There is no conflict of interest.
Figures
Similar articles
-
Distribution of ubiquilin 2 and TDP-43 aggregates throughout the CNS in UBQLN2 p.T487I-linked amyotrophic lateral sclerosis and frontotemporal dementia.Brain Pathol. 2024 May;34(3):e13230. doi: 10.1111/bpa.13230. Epub 2023 Dec 19. Brain Pathol. 2024. PMID: 38115557 Free PMC article.
-
TDP-43 as a potential biomarker for amyotrophic lateral sclerosis: a systematic review and meta-analysis.BMC Neurol. 2018 Jun 28;18(1):90. doi: 10.1186/s12883-018-1091-7. BMC Neurol. 2018. PMID: 29954341 Free PMC article.
-
Cerebrospinal Fluid TAR DNA-Binding Protein 43 Combined with Tau Proteins as a Candidate Biomarker for Amyotrophic Lateral Sclerosis and Frontotemporal Dementia Spectrum Disorders.Dement Geriatr Cogn Disord. 2017;44(3-4):144-152. doi: 10.1159/000478979. Epub 2017 Aug 23. Dement Geriatr Cogn Disord. 2017. PMID: 28848086
-
TDP-43 and Tau Oligomers in Alzheimer's Disease, Amyotrophic Lateral Sclerosis, and Frontotemporal Dementia.Neurobiol Dis. 2020 Dec;146:105130. doi: 10.1016/j.nbd.2020.105130. Epub 2020 Oct 14. Neurobiol Dis. 2020. PMID: 33065281 Free PMC article.
-
TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia.Lancet Neurol. 2010 Oct;9(10):995-1007. doi: 10.1016/S1474-4422(10)70195-2. Lancet Neurol. 2010. PMID: 20864052 Review.
Cited by
-
Aggregated SOD1 causes selective death of cultured human motor neurons.Sci Rep. 2018 Nov 6;8(1):16393. doi: 10.1038/s41598-018-34759-z. Sci Rep. 2018. PMID: 30401824 Free PMC article.
-
Promiscuous Roles of Autophagy and Proteasome in Neurodegenerative Proteinopathies.Int J Mol Sci. 2020 Apr 24;21(8):3028. doi: 10.3390/ijms21083028. Int J Mol Sci. 2020. PMID: 32344772 Free PMC article. Review.
-
Membrane nanotubes facilitate the propagation of inflammatory injury in the heart upon overactivation of the β-adrenergic receptor.Cell Death Dis. 2020 Nov 7;11(11):958. doi: 10.1038/s41419-020-03157-7. Cell Death Dis. 2020. PMID: 33161415 Free PMC article.
-
Membrane Charge Drives the Aggregation of TDP-43 Pathological Fragments.J Am Chem Soc. 2025 Apr 23;147(16):13577-13591. doi: 10.1021/jacs.5c00594. Epub 2025 Apr 8. J Am Chem Soc. 2025. PMID: 40198794 Free PMC article.
-
Tunneling Nanotubes in the Brain.Results Probl Cell Differ. 2024;73:203-227. doi: 10.1007/978-3-031-62036-2_10. Results Probl Cell Differ. 2024. PMID: 39242381 Review.
References
-
- Elman LB, McCluskey L, Grossman M. Motor neuron disease and frontotemporal lobar degeneration: a tale of two disorders linked to TDP-43. Neuro-Signals. 2008;16:85–90. - PubMed
-
- Collins M, Riascos D, Kovalik T, An J, Krupa K, Hood BL, Conrads TP, Renton AE, Traynor BJ, Bowser R. The RNA-binding motif 45 (RBM45) protein accumulates in inclusion bodies in amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with TDP-43 inclusions (FTLD-TDP) patients. Acta neuropathologica. 2012;124:717–732. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

