Segmentation and Image Analysis of Abnormal Lungs at CT: Current Approaches, Challenges, and Future Trends
- PMID: 26172351
- PMCID: PMC4521615
- DOI: 10.1148/rg.2015140232
Segmentation and Image Analysis of Abnormal Lungs at CT: Current Approaches, Challenges, and Future Trends
Abstract
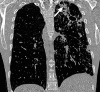
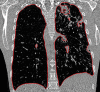
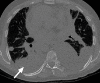
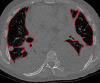
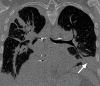
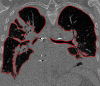
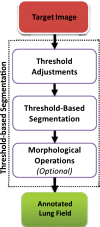
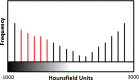
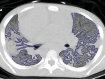
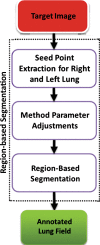
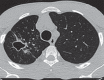
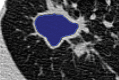
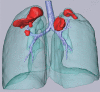
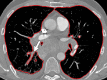
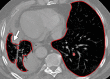
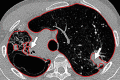
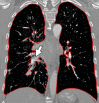
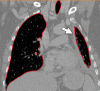
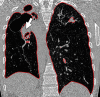
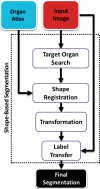
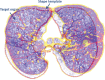
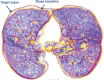
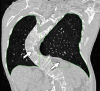
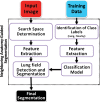
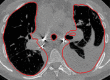
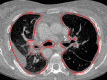
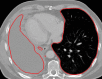
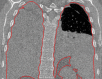
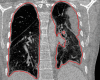
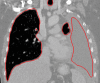
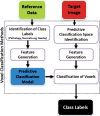
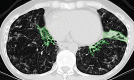
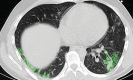
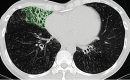
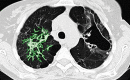
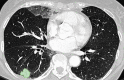
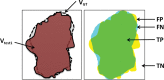
The computer-based process of identifying the boundaries of lung from surrounding thoracic tissue on computed tomographic (CT) images, which is called segmentation, is a vital first step in radiologic pulmonary image analysis. Many algorithms and software platforms provide image segmentation routines for quantification of lung abnormalities; however, nearly all of the current image segmentation approaches apply well only if the lungs exhibit minimal or no pathologic conditions. When moderate to high amounts of disease or abnormalities with a challenging shape or appearance exist in the lungs, computer-aided detection systems may be highly likely to fail to depict those abnormal regions because of inaccurate segmentation methods. In particular, abnormalities such as pleural effusions, consolidations, and masses often cause inaccurate lung segmentation, which greatly limits the use of image processing methods in clinical and research contexts. In this review, a critical summary of the current methods for lung segmentation on CT images is provided, with special emphasis on the accuracy and performance of the methods in cases with abnormalities and cases with exemplary pathologic findings. The currently available segmentation methods can be divided into five major classes: (a) thresholding-based, (b) region-based, (c) shape-based, (d) neighboring anatomy-guided, and (e) machine learning-based methods. The feasibility of each class and its shortcomings are explained and illustrated with the most common lung abnormalities observed on CT images. In an overview, practical applications and evolving technologies combining the presented approaches for the practicing radiologist are detailed.
©RSNA, 2015.
Figures






Similar articles
-
Automated segmentation of lungs with severe interstitial lung disease in CT.Med Phys. 2009 Oct;36(10):4592-9. doi: 10.1118/1.3222872. Med Phys. 2009. PMID: 19928090 Free PMC article.
-
Computer analysis of computed tomography scans of the lung: a survey.IEEE Trans Med Imaging. 2006 Apr;25(4):385-405. doi: 10.1109/TMI.2005.862753. IEEE Trans Med Imaging. 2006. PMID: 16608056 Review.
-
An edge-region force guided active shape approach for automatic lung field detection in chest radiographs.Comput Med Imaging Graph. 2012 Sep;36(6):452-63. doi: 10.1016/j.compmedimag.2012.04.005. Epub 2012 May 18. Comput Med Imaging Graph. 2012. PMID: 22608158
-
Computerized detection of diffuse lung disease in MDCT: the usefulness of statistical texture features.Phys Med Biol. 2009 Nov 21;54(22):6881-99. doi: 10.1088/0031-9155/54/22/009. Epub 2009 Oct 28. Phys Med Biol. 2009. PMID: 19864701
-
A review on lung boundary detection in chest X-rays.Int J Comput Assist Radiol Surg. 2019 Apr;14(4):563-576. doi: 10.1007/s11548-019-01917-1. Epub 2019 Feb 7. Int J Comput Assist Radiol Surg. 2019. PMID: 30730032 Free PMC article. Review.
Cited by
-
Quantitative Assessment of Parenchymal Involvement Using 3D Lung Model in Adolescent With Covid-19 Interstitial Pneumonia.Front Pediatr. 2020 Aug 5;8:453. doi: 10.3389/fped.2020.00453. eCollection 2020. Front Pediatr. 2020. PMID: 32850560 Free PMC article.
-
Segmentation of lung parenchyma in CT images using CNN trained with the clustering algorithm generated dataset.Biomed Eng Online. 2019 Jan 3;18(1):2. doi: 10.1186/s12938-018-0619-9. Biomed Eng Online. 2019. PMID: 30602393 Free PMC article.
-
Modality specific U-Net variants for biomedical image segmentation: a survey.Artif Intell Rev. 2022;55(7):5845-5889. doi: 10.1007/s10462-022-10152-1. Epub 2022 Mar 1. Artif Intell Rev. 2022. PMID: 35250146 Free PMC article.
-
Unsupervised CT Lung Image Segmentation of a Mycobacterium Tuberculosis Infection Model.Sci Rep. 2018 Jun 28;8(1):9802. doi: 10.1038/s41598-018-28100-x. Sci Rep. 2018. PMID: 29955159 Free PMC article.
-
Machine Learning in Ultrasound Computer-Aided Diagnostic Systems: A Survey.Biomed Res Int. 2018 Mar 4;2018:5137904. doi: 10.1155/2018/5137904. eCollection 2018. Biomed Res Int. 2018. PMID: 29687000 Free PMC article. Review.
References
-
- Hu S, Hoffman EA, Reinhardt JM. Automatic lung segmentation for accurate quantitation of volumetric X-ray CT images. IEEE Trans Med Imaging 2001;20(6):490–498. - PubMed
-
- Bagci U, Chen X, Udupa JK. Hierarchical scale-based multiobject recognition of 3-D anatomical structures. IEEE Trans Med Imaging 2012;31(3):777–789. - PubMed
-
- Marr D, Poggio T. A computational theory of human stereo vision. Proc R Soc Lond B Biol Sci 1979;204(1156):301–328. - PubMed
-
- Poggio T, Ullman S. Vision: are models of object recognition catching up with the brain? Ann N Y Acad Sci 2013;1305: 72–82. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

