Role of MR Imaging and FDG PET/CT in Selection and Follow-up of Patients Treated with Pelvic Exenteration for Gynecologic Malignancies
- PMID: 26172364
- PMCID: PMC4959795
- DOI: 10.1148/rg.2015140313
Role of MR Imaging and FDG PET/CT in Selection and Follow-up of Patients Treated with Pelvic Exenteration for Gynecologic Malignancies
Abstract
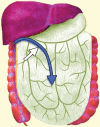
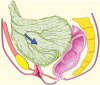
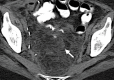
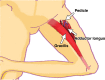
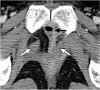
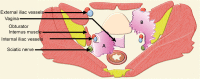
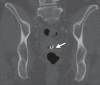
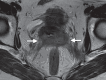
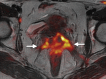
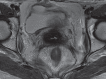
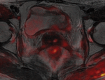
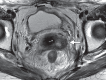
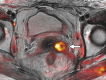
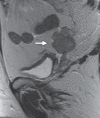
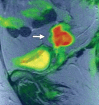
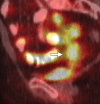
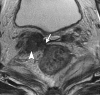
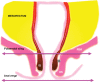
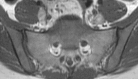
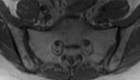
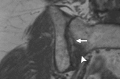
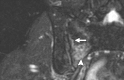
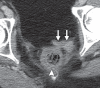
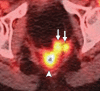
Pelvic exenteration (PE) is a radical surgical procedure used for the past 6 decades to treat locally advanced malignant diseases confined to the pelvis, particularly persistent or recurrent gynecologic cancers in the irradiated pelvis. The traditional surgical technique known as total PE consists of resection of all pelvic viscera followed by reconstruction. Depending on the tumor extent, the procedure can be tailored to remove only anterior or posterior structures, including the bladder (anterior exenteration) or rectum (posterior exenteration). Conversely, more extended pelvic resection can be performed if the pelvic sidewall is invaded by cancer. Preoperative imaging evaluation with magnetic resonance (MR) imaging and fluorine 18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) is central to establishing tumor resectability and therefore patient eligibility for the procedure. These imaging modalities complement each other in diagnosis of tumor recurrence and differentiation of persistent disease from posttreatment changes. MR imaging can accurately demonstrate local tumor extent and show adjacent organ invasion. FDG PET/CT is useful in excluding nodal and distant metastases. In addition, FDG PET/CT metrics may serve as predictive biomarkers for overall and disease-free survival. This pictorial review describes different types of exenterative surgical procedures and illustrates the central role of imaging in accurate patient selection, treatment planning, and postsurgical surveillance.
©RSNA, 2015.
Figures







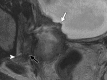
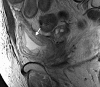
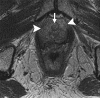
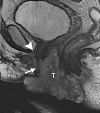
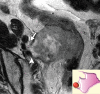
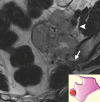

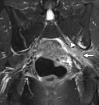
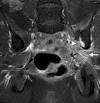
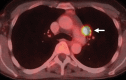
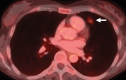

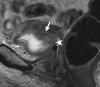
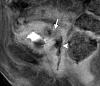
Similar articles
-
The value of 18F-FDG PET/CT in recurrent gynecologic malignancies prior to pelvic exenteration.Gynecol Oncol. 2013 Jun;129(3):586-592. doi: 10.1016/j.ygyno.2013.01.017. Epub 2013 Jan 29. Gynecol Oncol. 2013. PMID: 23369941 Free PMC article.
-
The role of imaging in pelvic exenteration for gynecological cancers.Br J Radiol. 2021 Sep 1;94(1125):20201460. doi: 10.1259/bjr.20201460. Epub 2021 May 7. Br J Radiol. 2021. PMID: 33960814 Free PMC article. Review.
-
A prospective study of the accuracy of 18Fluorodeoxyglucose positron emission tomography (18FDG PET) in identifying sites of metastasis prior to pelvic exenteration.Gynecol Oncol. 2007 Jul;106(1):177-80. doi: 10.1016/j.ygyno.2007.03.018. Epub 2007 May 2. Gynecol Oncol. 2007. PMID: 17477959
-
Overview of the Role of Imaging in Pelvic Exenteration.Radiographics. 2015 Jul-Aug;35(4):1286-94. doi: 10.1148/rg.2015140127. Radiographics. 2015. PMID: 26172363 Review.
-
Indications, techniques and outcomes for pelvic exenteration in gynecological malignancy.Curr Opin Oncol. 2014 Sep;26(5):514-20. doi: 10.1097/CCO.0000000000000109. Curr Opin Oncol. 2014. PMID: 25050632 Review.
Cited by
-
Staging of Cervical Cancer: A Practical Approach Using MRI and FDG PET.AJR Am J Roentgenol. 2023 Nov;221(5):633-648. doi: 10.2214/AJR.23.29003. Epub 2023 Jun 7. AJR Am J Roentgenol. 2023. PMID: 37459457 Free PMC article. Review.
-
Pelvic exenteration case series: A single surgeon's experience at one institution in Trinidad and Tobago.Int J Surg Case Rep. 2017;34:4-10. doi: 10.1016/j.ijscr.2017.03.006. Epub 2017 Mar 7. Int J Surg Case Rep. 2017. PMID: 28324802 Free PMC article.
-
Imaging in pelvic exenteration-a multidisciplinary practice guide from the ESGAR-SAR-ESUR-PelvEx collaborative group.Eur Radiol. 2025 May;35(5):2681-2691. doi: 10.1007/s00330-024-10940-z. Epub 2024 Aug 25. Eur Radiol. 2025. PMID: 39181949 Free PMC article.
-
MRI in the Evaluation of Locally Advanced Vulvar Cancer Treated with Chemoradiotherapy and Vulvar Cancer Recurrence: The 2021 Revision of FIGO Classification and the Need for Multidisciplinary Management.Cancers (Basel). 2022 Aug 9;14(16):3852. doi: 10.3390/cancers14163852. Cancers (Basel). 2022. PMID: 36010846 Free PMC article. Review.
-
MR guided high intensity focused ultrasound (MRgHIFU) for treating recurrent gynaecological tumours: a pilot feasibility study.Br J Radiol. 2019 Jun;92(1098):20181037. doi: 10.1259/bjr.20181037. Epub 2019 May 14. Br J Radiol. 2019. PMID: 31084495 Free PMC article.
References
-
- Höckel M, Dornhöfer N. Pelvic exenteration for gynaecological tumours: achievements and unanswered questions. Lancet Oncol 2006;7(10):837–847. - PubMed
-
- Brunschwig A. Complete excision of pelvic viscera for advanced carcinoma. Cancer 1948;1:177–183. - PubMed
-
- Berek JS, Howe C, Lagasse LD, Hacker NF. Pelvic exenteration for recurrent gynecologic malignancy: survival and morbidity analysis of the 45-year experience at UCLA. Gynecol Oncol 2005;99(1):153–159. - PubMed
-
- Baiocchi G, Guimaraes GC, Rosa Oliveira RA, et al. . Prognostic factors in pelvic exenteration for gynecological malignancies. Eur J Surg Oncol 2012;38(10):948–954. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

