A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache
- PMID: 26172390
- PMCID: PMC4501738
- DOI: 10.1371/journal.pone.0130733
A Comparative Effectiveness Meta-Analysis of Drugs for the Prophylaxis of Migraine Headache
Abstract
Objective: To compare the effectiveness and side effects of migraine prophylactic medications.
Design: We performed a network meta-analysis. Data were extracted independently in duplicate and quality was assessed using both the JADAD and Cochrane Risk of Bias instruments. Data were pooled and network meta-analysis performed using random effects models.
Data sources: PUBMED, EMBASE, Cochrane Trial Registry, bibliography of retrieved articles through 18 May 2014.
Eligibility criteria for selecting studies: We included randomized controlled trials of adults with migraine headaches of at least 4 weeks in duration.
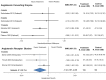
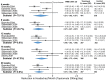
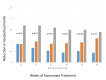
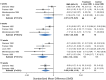
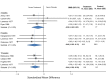
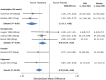
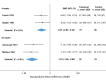
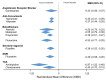
Results: Placebo controlled trials included alpha blockers (n = 9), angiotensin converting enzyme inhibitors (n = 3), angiotensin receptor blockers (n = 3), anticonvulsants (n = 32), beta-blockers (n = 39), calcium channel blockers (n = 12), flunarizine (n = 7), serotonin reuptake inhibitors (n = 6), serotonin norepinephrine reuptake inhibitors (n = 1) serotonin agonists (n = 9) and tricyclic antidepressants (n = 11). In addition there were 53 trials comparing different drugs. Drugs with at least 3 trials that were more effective than placebo for episodic migraines included amitriptyline (SMD: -1.2, 95% CI: -1.7 to -0.82), -flunarizine (-1.1 headaches/month (ha/month), 95% CI: -1.6 to -0.67), fluoxetine (SMD: -0.57, 95% CI: -0.97 to -0.17), metoprolol (-0.94 ha/month, 95% CI: -1.4 to -0.46), pizotifen (-0.43 ha/month, 95% CI: -0.6 to -0.21), propranolol (-1.3 ha/month, 95% CI: -2.0 to -0.62), topiramate (-1.1 ha/month, 95% CI: -1.9 to -0.73) and valproate (-1.5 ha/month, 95% CI: -2.1 to -0.8). Several effective drugs with less than 3 trials included: 3 ace inhibitors (enalapril, lisinopril, captopril), two angiotensin receptor blockers (candesartan, telmisartan), two anticonvulsants (lamotrigine, levetiracetam), and several beta-blockers (atenolol, bisoprolol, timolol). Network meta-analysis found amitriptyline to be better than several other medications including candesartan, fluoxetine, propranolol, topiramate and valproate and no different than atenolol, flunarizine, clomipramine or metoprolol.
Conclusion: Several drugs good evidence supporting efficacy. There is weak evidence supporting amitriptyline's superiority over some drugs. Selection of prophylactic medication should be tailored according to patient preferences, characteristics and side effect profiles.
Conflict of interest statement
Figures





References
-
- Rasmussen BK (2001) Epidemiology of headache. Cephalalgia 21: 774–777. - PubMed
-
- Wang SJ (2003) Epidemiology of migraine and other types of headache in Asia. Current Neurology & Neuroscience Reports 3: 104–108. - PubMed
-
- Falavigna A, Teles AR, Velho MC, Vedana VM, Silva RC, Mazzocchin T, et al. (2010) Prevalence and impact of headache in undergraduate students in Southern Brazil. Arq Neuropsiquiatr 68: 873–877. S0004-282X2010000600008 [pii]. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

