Phosphorylation of apoptosis repressor with caspase recruitment domain by protein kinase CK2 contributes to chemotherapy resistance by inhibiting doxorubicin induced apoptosis
- PMID: 26172393
- PMCID: PMC4695019
- DOI: 10.18632/oncotarget.4392
Phosphorylation of apoptosis repressor with caspase recruitment domain by protein kinase CK2 contributes to chemotherapy resistance by inhibiting doxorubicin induced apoptosis
Abstract
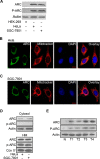
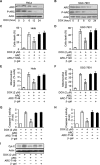
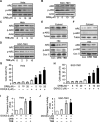
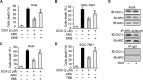
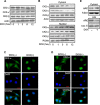
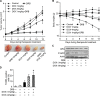
The development of cancer resistance to chemotherapy is the major obstacle to cancer therapy. Here, we identified that the phosphorylation of apoptosis repressor with caspase recruitment domain (ARC) at threonine 149 was essential to inhibit doxorubicin (DOX) induced apoptosis and mitochondrial fission in cancer cells. Our further study showed that casein kinase II (CK2) inhibitors could decrease the phosphorylation levels of ARC and make cancer cells sensitive to undergoing apoptosis. Furthermore, CK2α and CK2α', catalytic subunits of CK2, were observed to translocate into nuclear in cancer cells with the treatment of DOX. Finally, the synergistically therapeutic effect by combining DOX and CK2 inhibitor was confirmed in tumor xenograft model. Taken together, our results revealed that CK2-mediated phosphorylation of ARC contributed to chemotherapy resistance by inhibiting DOX induced apoptosis and combining DOX with CK2 inhibitor could induce apoptosis of cancer cells synergistically by down-regulating the phosphorylation of ARC. Therefore, development of new therapeutic strategies based on ARC and CK2, is promising for overcoming cancer resistance to chemotherapy.
Keywords: ARC; CK2; apoptosis; chemotherapy resistance; doxorubicin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Apoptosis repressor with caspase recruitment domain contributes to chemotherapy resistance by abolishing mitochondrial fission mediated by dynamin-related protein-1.Cancer Res. 2009 Jan 15;69(2):492-500. doi: 10.1158/0008-5472.CAN-08-2962. Cancer Res. 2009. PMID: 19147562
-
Influence of casein kinase II in tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human rhabdomyosarcoma cells.Clin Cancer Res. 2004 Oct 1;10(19):6650-60. doi: 10.1158/1078-0432.CCR-04-0576. Clin Cancer Res. 2004. PMID: 15475455
-
Induction of apoptosis by antisense CK2 in human prostate cancer xenograft model.Mol Cancer Res. 2004 Dec;2(12):712-21. Mol Cancer Res. 2004. PMID: 15634760
-
Repressing the activity of protein kinase CK2 releases the brakes on mitochondria-mediated apoptosis in cancer cells.Curr Drug Targets. 2011 Jun;12(6):902-8. doi: 10.2174/138945011795528831. Curr Drug Targets. 2011. PMID: 21269261 Review.
-
Protein kinase CK2 in health and disease: CK2: a key player in cancer biology.Cell Mol Life Sci. 2009 Jun;66(11-12):1858-67. doi: 10.1007/s00018-009-9154-y. Cell Mol Life Sci. 2009. PMID: 19387548 Free PMC article. Review.
Cited by
-
Role of microRNAs in regulation of doxorubicin and paclitaxel responses in lung tumor cells.Cell Div. 2023 Jul 21;18(1):11. doi: 10.1186/s13008-023-00093-8. Cell Div. 2023. PMID: 37480054 Free PMC article. Review.
-
MiR-194-5p enhances the sensitivity of nonsmall-cell lung cancer to doxorubicin through targeted inhibition of hypoxia-inducible factor-1.World J Surg Oncol. 2021 Jun 14;19(1):174. doi: 10.1186/s12957-021-02278-3. World J Surg Oncol. 2021. PMID: 34127010 Free PMC article.
-
A double-edged sword: role of apoptosis repressor with caspase recruitment domain (ARC) in tumorigenesis and ischaemia/reperfusion (I/R) injury.Apoptosis. 2023 Apr;28(3-4):313-325. doi: 10.1007/s10495-022-01802-4. Epub 2023 Jan 18. Apoptosis. 2023. PMID: 36652128 Review.
-
Role of protein kinase CK2 in antitumor drug resistance.J Exp Clin Cancer Res. 2019 Jul 5;38(1):287. doi: 10.1186/s13046-019-1292-y. J Exp Clin Cancer Res. 2019. PMID: 31277672 Free PMC article. Review.
-
Expression of apoptosis repressor with caspase recruitment domain (ARC) in familial adenomatous polyposis (FAP) adenomas and its correlation with DNA mismatch repair proteins, p53, Bcl-2, COX-2 and beta-catenin.Cell Commun Signal. 2021 Feb 12;19(1):15. doi: 10.1186/s12964-020-00702-x. Cell Commun Signal. 2021. PMID: 33579312 Free PMC article.
References
-
- Longley DB, Johnston PG. Molecular mechanisms of drug resistance. Journal of pathology. 2005;205:275–292. - PubMed
-
- Kang YJ, Zhou Z-X, Wang G-W, Buridi A, Klein JB. Suppression by metallothionein of doxorubicin-induced cardiomyocyte apoptosis through inhibition of p38 mitogenactivated protein kinases. Journal of Biological Chemistry. 2000;275:13690–13698. - PubMed
-
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 144:646–674. - PubMed
-
- Aas T, Borresen AL, Geisler S, SmithSorensen B, Johnsen H, Varhaug JE, Akslen LA, Lonning PE. Specific P53 mutations are associated with de novo resistance to doxorubicin in breast cancer patients. Nature Medicine. 1996;2:811–814. - PubMed
-
- Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nature Reviews Drug Discovery. 2011;10:111–126. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

