A Developmental Switch for Hebbian Plasticity
- PMID: 26172394
- PMCID: PMC4501799
- DOI: 10.1371/journal.pcbi.1004386
A Developmental Switch for Hebbian Plasticity
Abstract
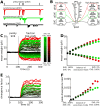
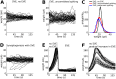
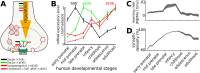
Hebbian forms of synaptic plasticity are required for the orderly development of sensory circuits in the brain and are powerful modulators of learning and memory in adulthood. During development, emergence of Hebbian plasticity leads to formation of functional circuits. By modeling the dynamics of neurotransmitter release during early postnatal cortical development we show that a developmentally regulated switch in vesicle exocytosis mode triggers associative (i.e. Hebbian) plasticity. Early in development spontaneous vesicle exocytosis (SVE), often considered as 'synaptic noise', is important for homogenization of synaptic weights and maintenance of synaptic weights in the appropriate dynamic range. Our results demonstrate that SVE has a permissive, whereas subsequent evoked vesicle exocytosis (EVE) has an instructive role in the expression of Hebbian plasticity. A timed onset for Hebbian plasticity can be achieved by switching from SVE to EVE and the balance between SVE and EVE can control the effective rate of Hebbian plasticity. We further show that this developmental switch in neurotransmitter release mode enables maturation of spike-timing dependent plasticity. A mis-timed or inadequate SVE to EVE switch may lead to malformation of brain networks thereby contributing to the etiology of neurodevelopmental disorders.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Synaptic plasticity: taming the beast.Nat Neurosci. 2000 Nov;3 Suppl:1178-83. doi: 10.1038/81453. Nat Neurosci. 2000. PMID: 11127835 Review.
-
Emergence and maintenance of modularity in neural networks with Hebbian and anti-Hebbian inhibitory STDP.PLoS Comput Biol. 2025 Apr 22;21(4):e1012973. doi: 10.1371/journal.pcbi.1012973. eCollection 2025 Apr. PLoS Comput Biol. 2025. PMID: 40262082 Free PMC article.
-
Synaptic Plasticity Forms and Functions.Annu Rev Neurosci. 2020 Jul 8;43:95-117. doi: 10.1146/annurev-neuro-090919-022842. Epub 2020 Feb 19. Annu Rev Neurosci. 2020. PMID: 32075520 Review.
-
Homeostatic Plasticity Achieved by Incorporation of Random Fluctuations and Soft-Bounded Hebbian Plasticity in Excitatory Synapses.Front Neural Circuits. 2016 Jun 1;10:42. doi: 10.3389/fncir.2016.00042. eCollection 2016. Front Neural Circuits. 2016. PMID: 27313513 Free PMC article.
-
Competitive Hebbian learning through spike-timing-dependent synaptic plasticity.Nat Neurosci. 2000 Sep;3(9):919-26. doi: 10.1038/78829. Nat Neurosci. 2000. PMID: 10966623
Cited by
-
Information transfer and recovery for the sense of touch.Cereb Cortex. 2025 Apr 1;35(4):bhaf073. doi: 10.1093/cercor/bhaf073. Cereb Cortex. 2025. PMID: 40197640 Free PMC article.
-
The role of spontaneous neurotransmission in synapse and circuit development.J Neurosci Res. 2018 Mar;96(3):354-359. doi: 10.1002/jnr.24154. Epub 2017 Oct 16. J Neurosci Res. 2018. PMID: 29034487 Free PMC article. Review.
-
Euchromatin histone methyltransferase 1 regulates cortical neuronal network development.Sci Rep. 2016 Oct 21;6:35756. doi: 10.1038/srep35756. Sci Rep. 2016. PMID: 27767173 Free PMC article.
-
A databank for intracellular electrophysiological mapping of the adult somatosensory cortex.Gigascience. 2018 Dec 1;7(12):giy147. doi: 10.1093/gigascience/giy147. Gigascience. 2018. PMID: 30521020 Free PMC article.
-
Adaptive Spike Threshold Enables Robust and Temporally Precise Neuronal Encoding.PLoS Comput Biol. 2016 Jun 15;12(6):e1004984. doi: 10.1371/journal.pcbi.1004984. eCollection 2016 Jun. PLoS Comput Biol. 2016. PMID: 27304526 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

