Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
- PMID: 26172439
- PMCID: PMC4501562
- DOI: 10.1371/journal.ppat.1005024
Vpu Exploits the Cross-Talk between BST2 and the ILT7 Receptor to Suppress Anti-HIV-1 Responses by Plasmacytoid Dendritic Cells
Abstract
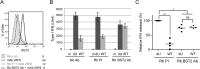
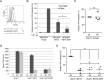
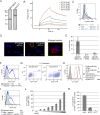
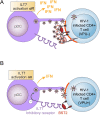
Plasmacytoid dendritic cells (pDCs) constitute a major source of type-I interferon (IFN-I) production during acute HIV infection. Their activation results primarily from TLR7-mediated sensing of HIV-infected cells. However, the interactions between HIV-infected T cells and pDCs that modulate this sensing process remain poorly understood. BST2/Tetherin is a restriction factor that inhibits HIV release by cross-linking virions onto infected cell surface. BST2 was also shown to engage the ILT7 pDC-specific inhibitory receptor and repress TLR7/9-mediated IFN-I production by activated pDCs. Here, we show that Vpu, the HIV-1 antagonist of BST2, suppresses TLR7-mediated IFN-I production by pDC through a mechanism that relies on the interaction of BST2 on HIV-producing cells with ILT7. Even though Vpu downregulates surface BST2 as a mean to counteract the restriction on HIV-1 release, we also find that the viral protein re-locates remaining BST2 molecules outside viral assembly sites where they are free to bind and activate ILT7 upon cell-to-cell contact. This study shows that through a targeted regulation of surface BST2, Vpu promotes HIV-1 release and limits pDC antiviral responses upon sensing of infected cells. This mechanism of innate immune evasion is likely to be important for an efficient early viral dissemination during acute infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Differential Control of BST2 Restriction and Plasmacytoid Dendritic Cell Antiviral Response by Antagonists Encoded by HIV-1 Group M and O Strains.J Virol. 2016 Oct 28;90(22):10236-10246. doi: 10.1128/JVI.01131-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581991 Free PMC article.
-
Contribution of the Cytoplasmic Determinants of Vpu to the Expansion of Virus-Containing Compartments in HIV-1-Infected Macrophages.J Virol. 2019 May 15;93(11):e00020-19. doi: 10.1128/JVI.00020-19. Print 2019 Jun 1. J Virol. 2019. PMID: 30867316 Free PMC article.
-
Emerging role of the host restriction factor tetherin in viral immune sensing.J Mol Biol. 2013 Dec 13;425(24):4956-64. doi: 10.1016/j.jmb.2013.09.029. Epub 2013 Sep 26. J Mol Biol. 2013. PMID: 24075872 Review.
-
Ig-like transcript 7, but not bone marrow stromal cell antigen 2 (also known as HM1.24, tetherin, or CD317), modulates plasmacytoid dendritic cell function in primary human blood leukocytes.J Immunol. 2013 Mar 15;190(6):2622-30. doi: 10.4049/jimmunol.1202391. Epub 2013 Feb 11. J Immunol. 2013. PMID: 23401591 Free PMC article.
-
Role of the endosomal ESCRT machinery in HIV-1 Vpu-induced down-regulation of BST2/tetherin.Curr HIV Res. 2012 Jun;10(4):315-20. doi: 10.2174/157016212800792414. Curr HIV Res. 2012. PMID: 22524180 Review.
Cited by
-
Plasma Membrane-Associated Restriction Factors and Their Counteraction by HIV-1 Accessory Proteins.Cells. 2019 Sep 2;8(9):1020. doi: 10.3390/cells8091020. Cells. 2019. PMID: 31480747 Free PMC article. Review.
-
Interplay between Intrinsic and Innate Immunity during HIV Infection.Cells. 2019 Aug 17;8(8):922. doi: 10.3390/cells8080922. Cells. 2019. PMID: 31426525 Free PMC article. Review.
-
Limiting Respiratory Viral Infection by Targeting Antiviral and Immunological Functions of BST-2/Tetherin: Knowledge and Gaps.Bioessays. 2018 Oct;40(10):e1800086. doi: 10.1002/bies.201800086. Epub 2018 Aug 16. Bioessays. 2018. PMID: 30113067 Free PMC article. Review.
-
Remodeling of the Host Cell Plasma Membrane by HIV-1 Nef and Vpu: A Strategy to Ensure Viral Fitness and Persistence.Viruses. 2016 Mar 3;8(3):67. doi: 10.3390/v8030067. Viruses. 2016. PMID: 26950141 Free PMC article. Review.
-
HIV-1 Vpu Promotes Phagocytosis of Infected CD4+ T Cells by Macrophages through Downregulation of CD47.mBio. 2021 Aug 31;12(4):e0192021. doi: 10.1128/mBio.01920-21. Epub 2021 Aug 24. mBio. 2021. PMID: 34425695 Free PMC article.
References
-
- Campillo-Gimenez L, Laforge M, Fay M, Brussel A, Cumont MC, et al. (2010) Nonpathogenesis of simian immunodeficiency virus infection is associated with reduced inflammation and recruitment of plasmacytoid dendritic cells to lymph nodes, not to lack of an interferon type I response, during the acute phase. J Virol 84: 1838–1846. 10.1128/JVI.01496-09 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

