The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome
- PMID: 26172456
- PMCID: PMC5042064
- DOI: 10.1002/path.4584
The oncogenic potential of BK-polyomavirus is linked to viral integration into the human genome
Abstract
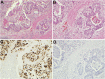
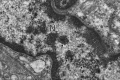
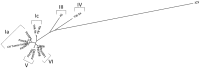
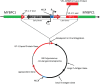
It has been suggested that BK-polyomavirus is linked to oncogenesis via high expression levels of large T-antigen in some urothelial neoplasms arising following kidney transplantation. However, a causal association between BK-polyomavirus, large T-antigen expression and oncogenesis has never been demonstrated in humans. Here we describe an investigation using high-throughput sequencing of tumour DNA obtained from an urothelial carcinoma arising in a renal allograft. We show that a novel BK-polyomavirus strain, named CH-1, is integrated into exon 26 of the myosin-binding protein C1 gene (MYBPC1) on chromosome 12 in tumour cells but not in normal renal cells. Integration of the BK-polyomavirus results in a number of discrete alterations in viral gene expression, including: (a) disruption of VP1 protein expression and robust expression of large T-antigen; (b) preclusion of viral replication; and (c) deletions in the non-coding control region (NCCR), with presumed alterations in promoter feedback loops. Viral integration disrupts one MYBPC1 gene copy and likely alters its expression. Circular episomal BK-polyomavirus gene sequences are not found, and the renal allograft shows no productive polyomavirus infection or polyomavirus nephropathy. These findings support the hypothesis that integration of polyomaviruses is essential to tumourigenesis. It is likely that dysregulation of large T-antigen, with persistent over-expression in non-lytic cells, promotes cell growth, genetic instability and neoplastic transformation.
Keywords: BK virus; carcinoma; cell transformation, neoplastic; kidney transplantation; large T Antigen; oncogenesis; polyomavirus; polyomavirus nephropathy; virus integration.
© 2015 The Authors. The Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland.
Figures

Similar articles
-
Donor-derived, metastatic urothelial cancer after kidney transplantation associated with a potentially oncogenic BK polyomavirus.J Pathol. 2018 Mar;244(3):265-270. doi: 10.1002/path.5012. Epub 2018 Feb 1. J Pathol. 2018. PMID: 29205775
-
BK Polyomavirus Genomic Integration and Large T Antigen Expression: Evolving Paradigms in Human Oncogenesis.Am J Transplant. 2017 Jun;17(6):1674-1680. doi: 10.1111/ajt.14191. Epub 2017 Feb 21. Am J Transplant. 2017. PMID: 28039910
-
Occurrence and regression of BK polyomavirus associated carcinoma: a clinical and next-generation sequencing study.Clin Sci (Lond). 2018 Aug 30;132(16):1753-1763. doi: 10.1042/CS20180443. Print 2018 Aug 31. Clin Sci (Lond). 2018. PMID: 30026258
-
BK virus-associated urinary bladder carcinoma in transplant recipients: report of 2 cases, review of the literature, and proposed pathogenetic model.Hum Pathol. 2013 May;44(5):908-17. doi: 10.1016/j.humpath.2012.09.019. Epub 2013 Jan 11. Hum Pathol. 2013. PMID: 23317548 Review.
-
BK Polyomavirus-Associated Urothelial Carcinoma of the Bladder with a Background of BK Polyomavirus Nephropathy in a Kidney Transplant Recipient.Nephron. 2023;147 Suppl 1:53-60. doi: 10.1159/000531822. Epub 2023 Aug 2. Nephron. 2023. PMID: 37531946 Review.
Cited by
-
Survey for human polyomaviruses in cancer.JCI Insight. 2016 Feb;1(2):e85562. doi: 10.1172/jci.insight.85562. Epub 2016 Feb 25. JCI Insight. 2016. PMID: 27034991 Free PMC article.
-
High Incidence and Early Onset of Urinary Tract Cancers in Patients with BK Polyomavirus Associated Nephropathy.Viruses. 2021 Mar 14;13(3):476. doi: 10.3390/v13030476. Viruses. 2021. PMID: 33799453 Free PMC article.
-
Human polyomaviruses and cancer: an overview.Clinics (Sao Paulo). 2018 Oct 11;73(suppl 1):e558s. doi: 10.6061/clinics/2018/e558s. Clinics (Sao Paulo). 2018. PMID: 30328951 Free PMC article. Review.
-
Metastatic Urothelial Carcinoma from Transplanted Kidney with Complete Response to an Immune Checkpoint Inhibitor.Case Rep Urol. 2020 Dec 23;2020:8881841. doi: 10.1155/2020/8881841. eCollection 2020. Case Rep Urol. 2020. PMID: 33425425 Free PMC article.
-
The case for BK polyomavirus as a cause of bladder cancer.Curr Opin Virol. 2019 Dec;39:8-15. doi: 10.1016/j.coviro.2019.06.009. Epub 2019 Jul 20. Curr Opin Virol. 2019. PMID: 31336246 Free PMC article. Review.
References
-
- Kotton CN, Fishman JA. Viral infection in the renal transplant recipient. J Am Soc Nephrol 2005; 16: 1758–1774. - PubMed
-
- Birkeland SA, Storm HH, Lamm LU, et al. Cancer risk after renal transplantation in the Nordic countries, 1964–1986. Int J Cancer 1995; 60: 183–189. - PubMed
-
- Buzzeo BD, Heisey DM, Messing EM. Bladder cancer in renal transplant recipients. Urology 1997; 50: 525–528. - PubMed
-
- Vajdic CM, McDonald SP, McCredie MR, et al. Cancer incidence before and after kidney transplantation. J Am Med Assoc 2006; 296: 2823–2831. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

