IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas
- PMID: 26173023
- PMCID: PMC4501840
- DOI: 10.1371/journal.pone.0133152
IGF1R- and ROR1-Specific CAR T Cells as a Potential Therapy for High Risk Sarcomas
Abstract
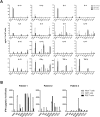
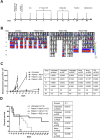
Patients with metastatic or recurrent and refractory sarcomas have a dismal prognosis. Therefore, new targeted therapies are urgently needed. This study was designed to evaluate chimeric antigen receptor (CAR) T cells targeting the type I insulin-like growth factor receptor (IGF1R) or tyrosine kinase-like orphan receptor 1 (ROR1) molecules for their therapeutic potential against sarcomas. Here, we report that IGF1R (15/15) and ROR1 (11/15) were highly expressed in sarcoma cell lines including Ewing sarcoma, osteosarcoma, alveolar or embryonal rhabdomyosarcoma, and fibrosarcoma. IGF1R and ROR1 CAR T cells derived from eight healthy donors using the Sleeping Beauty (SB) transposon system were cytotoxic against sarcoma cells and produced high levels of IFN-γ, TNF-α and IL-13 in an antigen-specific manner. IGF1R and ROR1 CAR T cells generated from three sarcoma patients released significant amounts of IFN-γ in response to sarcoma stimulation. The adoptive transfer of IGF1R and ROR1 CAR T cells derived from a sarcoma patient significantly reduced tumor growth in pre-established, systemically disseminated and localized osteosarcoma xenograft models in NSG mice. Infusion of IGF1R and ROR1 CAR T cells also prolonged animal survival in a localized sarcoma model using NOD/scid mice. Our data indicate that both IGF1R and ROR1 can be effectively targeted by SB modified CAR T cells and that such CAR T cells may be useful in the treatment of high risk sarcoma patients.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

