Age-related changes in the topological organization of the white matter structural connectome across the human lifespan
- PMID: 26173024
- PMCID: PMC6869038
- DOI: 10.1002/hbm.22877
Age-related changes in the topological organization of the white matter structural connectome across the human lifespan
Abstract
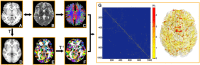
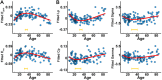
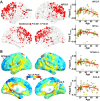
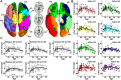
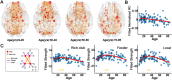
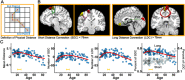
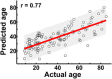
Lifespan is a dynamic process with remarkable changes in brain structure and function. Previous neuroimaging studies have indicated age-related microstructural changes in specific white matter tracts during development and aging. However, the age-related alterations in the topological architecture of the white matter structural connectome across the human lifespan remain largely unknown. Here, a cohort of 113 healthy individuals (ages 9-85) with both diffusion and structural MRI acquisitions were examined. For each participant, the high-resolution white matter structural networks were constructed by deterministic fiber tractography among 1024 parcellation units and were quantified with graph theoretical analyses. The global network properties, including network strength, cost, topological efficiency, and robustness, followed an inverted U-shaped trajectory with a peak age around the third decade. The brain areas with the most significantly nonlinear changes were located in the prefrontal and temporal cortices. Different brain regions exhibited heterogeneous trajectories: the posterior cingulate and lateral temporal cortices displayed prolonged maturation/degeneration compared with the prefrontal cortices. Rich-club organization was evident across the lifespan, whereas hub integration decreased linearly with age, especially accompanied by the loss of frontal hubs and their connections. Additionally, age-related changes in structural connections were predominantly located within and between the prefrontal and temporal modules. Finally, based on the graph metrics of structural connectome, accurate predictions of individual age were obtained (r = 0.77). Together, the data indicated a dynamic topological organization of the brain structural connectome across human lifespan, which may provide possible structural substrates underlying functional and cognitive changes with age.
Keywords: brain network; diffusion MRI; fiber tractography; graph theory; lifespan; white matter.
© 2015 Wiley Periodicals, Inc.
Figures
References
-
- Akaike H (1974): A new look at statistical‐model identification. IEEE Trans Automat Control 19:716–723.
-
- Alvarez JA, Emory E (2006): Executive function and the frontal lobes: A meta‐analytic review. Neuropsychol Rev 16:17–42. - PubMed
-
- Ashburner J, Friston KJ (2005): Unified segmentation. NeuroImage 26:839–51. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

