The molecular evolution of spiggin nesting glue in sticklebacks
- PMID: 26173374
- PMCID: PMC4989455
- DOI: 10.1111/mec.13317
The molecular evolution of spiggin nesting glue in sticklebacks
Abstract
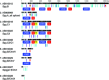
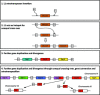
Gene duplication and subsequent divergence can lead to the evolution of new functions and lineage-specific traits. In sticklebacks, the successive duplication of a mucin gene (MUC19) into a tandemly arrayed, multigene family has enabled the production of copious amounts of 'spiggin', a secreted adhesive protein essential for nest construction. Here, we examine divergence between spiggin genes among three-spined sticklebacks (Gasterosteus aculeatus) from ancestral marine and derived freshwater populations, and propose underpinning gene duplication mechanisms. Sanger sequencing revealed substantial diversity among spiggin transcripts, including alternatively spliced variants and interchromosomal spiggin chimeric genes. Comparative analysis of the sequenced transcripts and all other spiggin genes in the public domain support the presence of three main spiggin lineages (spiggin A, spiggin B and spiggin C) with further subdivisions within spiggin B (B1, B2) and spiggin C (C1, C2). Spiggin A had diverged least from the ancestral MUC19, while the spiggin C duplicates had diversified most substantially. In silico translations of the spiggin gene open reading frames predicted that spiggins A and B are secreted as long mucin-like polymers, while spiggins C1 and C2 are secreted as short monomers, with putative antimicrobial properties. We propose that diversification of duplicated spiggin genes has facilitated local adaptation of spiggin to a range of aquatic habitats.
Keywords: Gasterosteus aculeatus; adaptation; gene duplication; gene family; nest building; retrotransposon.
© 2015 John Wiley & Sons Ltd.
Figures





References
-
- Bansil R, Stanley E, LaMont JT (1995) Mucin biophysics. Annual Review of Physiology, 57, 635–657. - PubMed
-
- Barber I, Nairn D, Huntingford FA (2001) Nests as ornaments: revealing construction by male sticklebacks. Behavioral Ecology, 12, 390–396.
-
- Bell MA, Foster SA (1994) Introduction to the evolutionary biology of the threespine stickleback In: The Evolutionary Biology of the Threespine Stickleback (eds Bell MA, Foster SA.), pp. 297–344. Oxford University Press, Oxford.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

