Fell-Muir Lecture: Heparan sulphate and the art of cell regulation: a polymer chain conducts the protein orchestra
- PMID: 26173450
- PMCID: PMC4561558
- DOI: 10.1111/iep.12135
Fell-Muir Lecture: Heparan sulphate and the art of cell regulation: a polymer chain conducts the protein orchestra
Abstract
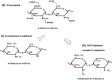
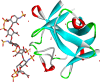
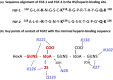
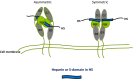
Heparan sulphate (HS) sits at the interface of the cell and the extracellular matrix. It is a member of the glycosaminoglycan family of anionic polysaccharides with unique structural features designed for protein interaction and regulation. Its client proteins include soluble effectors (e.g. growth factors, morphogens, chemokines), membrane receptors and cell adhesion proteins such as fibronectin, fibrillin and various types of collagen. The protein-binding properties of HS, together with its strategic positioning in the pericellular domain, are indicative of key roles in mediating the flow of regulatory signals between cells and their microenvironment. The control of transmembrane signalling is a fundamental element in the complex biology of HS. It seems likely that, in some way, HS orchestrates diverse signalling pathways to facilitate information processing inside the cell. A dictionary definition of an orchestra is 'a large group of musicians who play together on various instruments …' to paraphrase, the HS orchestra is 'a large group of proteins that play together on various receptors'. HS conducts this orchestra to ensure that proteins hit the right notes on their receptors but, in the manner of a true conductor, does it also set 'the musical pulse' and create rhythm and harmony attractive to the cell? This is too big a question to answer but fun to think about as you read this review.
Keywords: glycosaminoglycan; heparan sulphate; heparan sulphate/heparin.
© 2015 The Authors. International Journal of Experimental Pathology published by John Wiley & Sons Ltd on behalf of Company of the International Journal of Experimental Pathology (CIJEP).
Figures





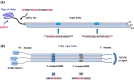
References
-
- Ai X, Kitazawa T, Do AT, Kusche-Gullberg M, Labosky PA. Emerson CP., Jr SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development. 2007;134:3327–3338. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

