Evidence for a novel Kit adhesion domain mediating human mast cell adhesion to structural airway cells
- PMID: 26173671
- PMCID: PMC4501212
- DOI: 10.1186/s12931-015-0245-z
Evidence for a novel Kit adhesion domain mediating human mast cell adhesion to structural airway cells
Abstract
Background: Human lung mast cells (HLMCs) infiltrate the airway epithelium and airway smooth muscle (ASM) in asthmatic airways. The mechanism of HLMC adhesion to both cell types is only partly defined, and adhesion is not inhibited by function-blocking anti-Kit and anti-stem cell factor (SCF) antibodies. Our aim was to identify adhesion molecules expressed by human mast cells that mediate adhesion to human ASM cells (HASMCs) and human airway epithelial cells.
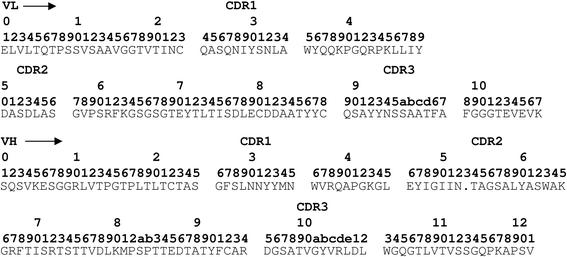
Methods: We used phage-display to isolate single chain Fv (scFv) antibodies with adhesion-blocking properties from rabbits immunised with HLMC and HMC-1 membrane proteins.
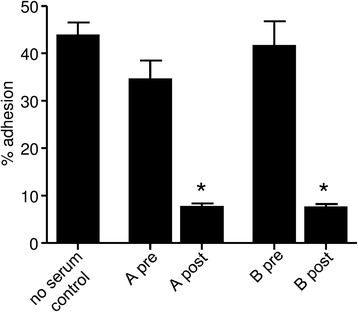
Results: Post-immune rabbit serum labelled HLMCs in flow cytometry and inhibited their adhesion to human BEAS-2B epithelial cells. Mast cell-specific scFvs were identified which labelled mast cells but not Jurkat cells by flow cytometry. Of these, one scFv (A1) consistently inhibited mast cell adhesion to HASMCs and BEAS-2B epithelial cells by about 30 %. A1 immunoprecipitated Kit (CD117) from HMC-1 lysates and bound to a human Kit-expressing mouse mast cell line, but did not interfere with SCF-dependent Kit signalling.
Conclusion: Kit contributes to human mast cell adhesion to human airway epithelial cells and HASMCs, but may utilise a previously unidentified adhesion domain that lies outside the SCF binding site. Targeting this adhesion pathway might offer a novel approach for the inhibition of mast cell interactions with structural airway cells, without detrimental effects on Kit signalling in other tissues.
Figures






Similar articles
-
CADM1 is a key receptor mediating human mast cell adhesion to human lung fibroblasts and airway smooth muscle cells.PLoS One. 2013 Apr 19;8(4):e61579. doi: 10.1371/journal.pone.0061579. Print 2013. PLoS One. 2013. PMID: 23620770 Free PMC article.
-
Bidirectional Counterregulation of Human Lung Mast Cell and Airway Smooth Muscle β2 Adrenoceptors.J Immunol. 2016 Jan 1;196(1):55-63. doi: 10.4049/jimmunol.1402232. Epub 2015 Nov 25. J Immunol. 2016. PMID: 26608913 Free PMC article.
-
Human airway smooth muscle promotes human lung mast cell survival, proliferation, and constitutive activation: cooperative roles for CADM1, stem cell factor, and IL-6.J Immunol. 2008 Aug 15;181(4):2772-80. doi: 10.4049/jimmunol.181.4.2772. J Immunol. 2008. PMID: 18684968 Free PMC article.
-
Stem cell factor expression, mast cells and inflammation in asthma.Fundam Clin Pharmacol. 2006 Feb;20(1):21-39. doi: 10.1111/j.1472-8206.2005.00390.x. Fundam Clin Pharmacol. 2006. PMID: 16448392 Review.
-
Stem cell factor and its receptor c-Kit as targets for inflammatory diseases.Eur J Pharmacol. 2006 Mar 8;533(1-3):327-40. doi: 10.1016/j.ejphar.2005.12.067. Epub 2006 Feb 17. Eur J Pharmacol. 2006. PMID: 16483568 Review.
Cited by
-
From rabbit antibody repertoires to rabbit monoclonal antibodies.Exp Mol Med. 2017 Mar 24;49(3):e305. doi: 10.1038/emm.2017.23. Exp Mol Med. 2017. PMID: 28336958 Free PMC article. Review.
-
Effect of miR-506-3p on Proliferation and Apoptosis of Airway Smooth Muscle Cells in Asthmatic Mice by Regulating CCL2 Gene Expression and Mediating TLR4/NF-κB Signaling Pathway Activation.Mol Biotechnol. 2021 May;63(5):410-423. doi: 10.1007/s12033-021-00309-8. Epub 2021 Feb 27. Mol Biotechnol. 2021. PMID: 33638773
-
Advances in the Production and Batch Reformatting of Phage Antibody Libraries.Mol Biotechnol. 2019 Nov;61(11):801-815. doi: 10.1007/s12033-019-00207-0. Mol Biotechnol. 2019. PMID: 31468301 Free PMC article. Review.
References
-
- Bradding P, Roberts JA, Britten KM, Montefort S, Djukanovic R, Mueller R, Heusser CH, Howarth PH, Holgate ST. Interleukin-4, −5, and −6 and tumor necrosis factor-alpha in normal and asthmatic airways: evidence for the human mast cell as a source of these cytokines. Am J Respir Cell Mol Biol. 1994;10:471–80. doi: 10.1165/ajrcmb.10.5.8179909. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

