A Single Host-Derived Glycan Impacts Key Regulatory Nodes of Symbiont Metabolism in a Coevolved Mutualism
- PMID: 26173698
- PMCID: PMC4502230
- DOI: 10.1128/mBio.00811-15
A Single Host-Derived Glycan Impacts Key Regulatory Nodes of Symbiont Metabolism in a Coevolved Mutualism
Abstract
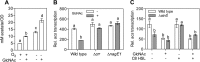
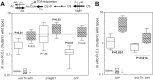
Most animal-microbe mutualistic associations are characterized by nutrient exchange between the partners. When the host provides the nutrients, it can gain the capacity to shape its microbial community, control the stability of the interaction, and promote its health and fitness. Using the bioluminescent squid-vibrio model, we demonstrate how a single host-derived glycan, chitin, regulates the metabolism of Vibrio fischeri at key points in the development and maintenance of the symbiosis. We first characterized the pathways for catabolism of chitin sugars by V. fischeri, demonstrating that the Ccr-dependent phosphoenolpyruvate-pyruvate phosphotransferase system (PTS) prioritizes transport of these sugars in V. fischeri by blocking the uptake of non-PTS carbohydrates, such as glycerol. Next, we found that PTS transport of chitin sugars into the bacterium shifted acetate homeostasis toward a net excretion of acetate and was sufficient to override an activation of the acetate switch by AinS-dependent quorum sensing. Finally, we showed that catabolism of chitin sugars decreases the rate of cell-specific oxygen consumption. Collectively, these three metabolic functions define a physiological shift that favors fermentative growth on chitin sugars and may support optimal symbiont luminescence, the functional basis of the squid-vibrio mutualism.
Importance: Host-derived glycans have recently emerged as a link between symbiont nutrition and innate immune function. Unfortunately, the locations at which microbes typically access host-derived glycans are inaccessible to experimentation and imaging, and they take place in the context of diverse microbe-microbe interactions, creating a complex symbiotic ecology. Here we describe the metabolic state of a single microbial symbiont in a natural association with its coevolved host and, by doing so, infer key points at which a host-controlled tissue environment might regulate the physiological state of its symbionts. We show that the presence of a regulatory glycan is sufficient to shift symbiont carbohydrate catabolism, acetate homeostasis, and oxygen consumption.
Copyright © 2015 Pan et al.
Figures



Similar articles
-
The chemistry of negotiation: rhythmic, glycan-driven acidification in a symbiotic conversation.Proc Natl Acad Sci U S A. 2015 Jan 13;112(2):566-71. doi: 10.1073/pnas.1418580112. Epub 2014 Dec 30. Proc Natl Acad Sci U S A. 2015. PMID: 25550509 Free PMC article.
-
Bacterial Quorum-Sensing Regulation Induces Morphological Change in a Key Host Tissue during the Euprymna scolopes-Vibrio fischeri Symbiosis.mBio. 2021 Oct 26;12(5):e0240221. doi: 10.1128/mBio.02402-21. Epub 2021 Sep 28. mBio. 2021. PMID: 34579565 Free PMC article.
-
Squid-derived chitin oligosaccharides are a chemotactic signal during colonization by Vibrio fischeri.Appl Environ Microbiol. 2012 Jul;78(13):4620-6. doi: 10.1128/AEM.00377-12. Epub 2012 Apr 20. Appl Environ Microbiol. 2012. PMID: 22522684 Free PMC article.
-
Host/microbe interactions revealed through "omics" in the symbiosis between the Hawaiian bobtail squid Euprymna scolopes and the bioluminescent bacterium Vibrio fischeri.Biol Bull. 2012 Aug;223(1):103-11. doi: 10.1086/BBLv223n1p103. Biol Bull. 2012. PMID: 22983036 Review.
-
Vibrio fischeri metabolism: symbiosis and beyond.Adv Microb Physiol. 2012;61:37-68. doi: 10.1016/B978-0-12-394423-8.00002-0. Adv Microb Physiol. 2012. PMID: 23046951 Review.
Cited by
-
Transcriptional characterization of Vibrio fischeri during colonization of juvenile Euprymna scolopes.Environ Microbiol. 2017 May;19(5):1845-1856. doi: 10.1111/1462-2920.13684. Epub 2017 Mar 21. Environ Microbiol. 2017. PMID: 28152560 Free PMC article.
-
A lasting symbiosis: how Vibrio fischeri finds a squid partner and persists within its natural host.Nat Rev Microbiol. 2021 Oct;19(10):654-665. doi: 10.1038/s41579-021-00557-0. Epub 2021 Jun 4. Nat Rev Microbiol. 2021. PMID: 34089008 Free PMC article. Review.
-
Rethinking the roles of CRP, cAMP, and sugar-mediated global regulation in the Vibrionaceae.Curr Genet. 2016 Feb;62(1):39-45. doi: 10.1007/s00294-015-0508-8. Epub 2015 Jul 28. Curr Genet. 2016. PMID: 26215147 Review.
-
Model-enabled gene search (MEGS) allows fast and direct discovery of enzymatic and transport gene functions in the marine bacterium Vibrio fischeri.J Biol Chem. 2017 Jun 16;292(24):10250-10261. doi: 10.1074/jbc.M116.763193. Epub 2017 Apr 26. J Biol Chem. 2017. PMID: 28446608 Free PMC article.
-
Generation and validation of a versatile inducible multiplex CRISPRi system to examine bacterial regulation in the Euprymna-Vibrio fischeri symbiosis.Arch Microbiol. 2025 May 17;207(7):147. doi: 10.1007/s00203-025-04354-8. Arch Microbiol. 2025. PMID: 40380978 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
