Mutant KRAS associated malic enzyme 1 expression is a predictive marker for radiation therapy response in non-small cell lung cancer
- PMID: 26173780
- PMCID: PMC4502640
- DOI: 10.1186/s13014-015-0457-x
Mutant KRAS associated malic enzyme 1 expression is a predictive marker for radiation therapy response in non-small cell lung cancer
Abstract
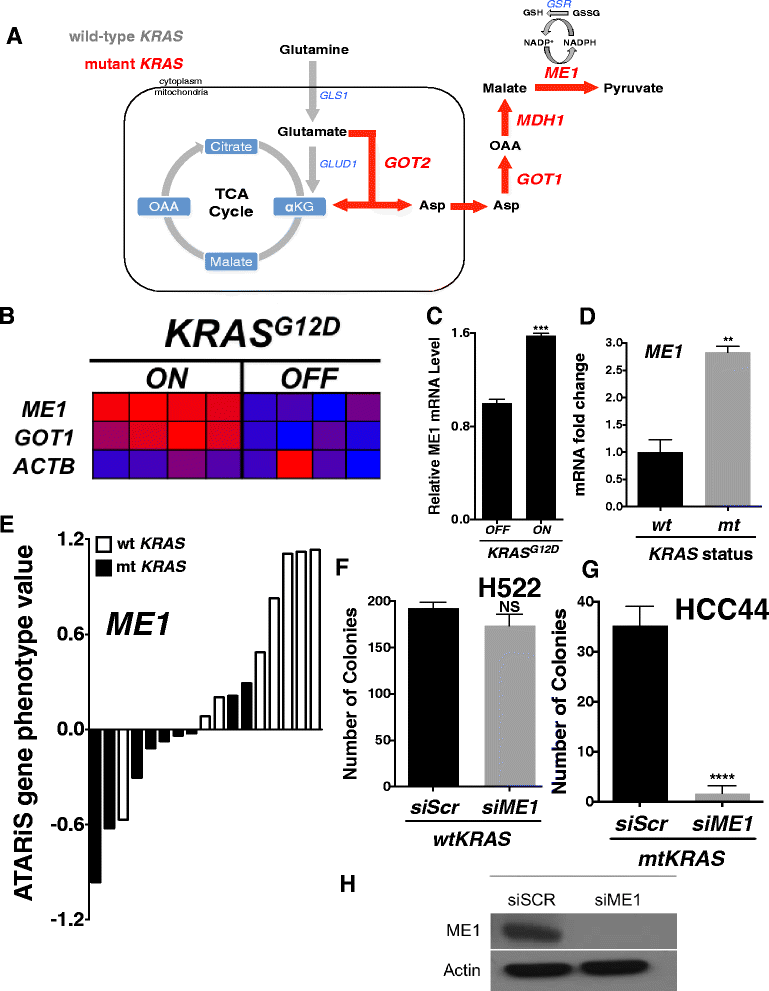
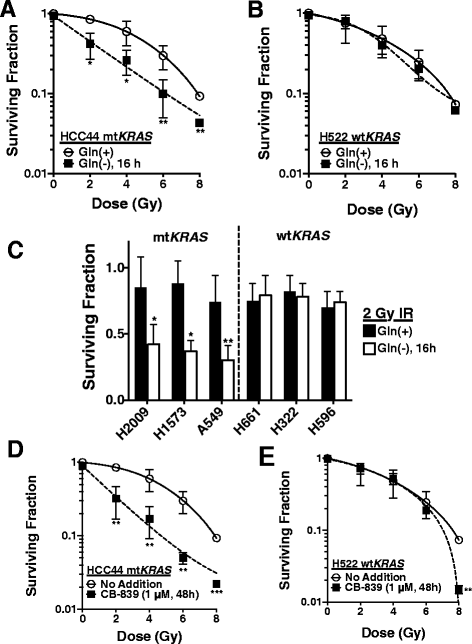
Background: Advanced non-small cell lung cancer (NSCLC) is an aggressive tumor that is treated with a combination of chemotherapy and radiation if the patient is not a candidate for surgery. Predictive biomarkers for response to radiotherapy are lacking in this patient population, making it a non-tailored therapy regimen with unknown outcome. Twenty to 30 % of NSCLC harbor an activating mutation in KRAS that may confer radioresistance. We hypothesized that mutant KRAS can regulate glutamine metabolism genes in NSCLC and maintain tumor redox balance through transamination reactions that generate cytosolic NADPH via malic enzyme 1 (ME1), which may contribute to radioresistance.
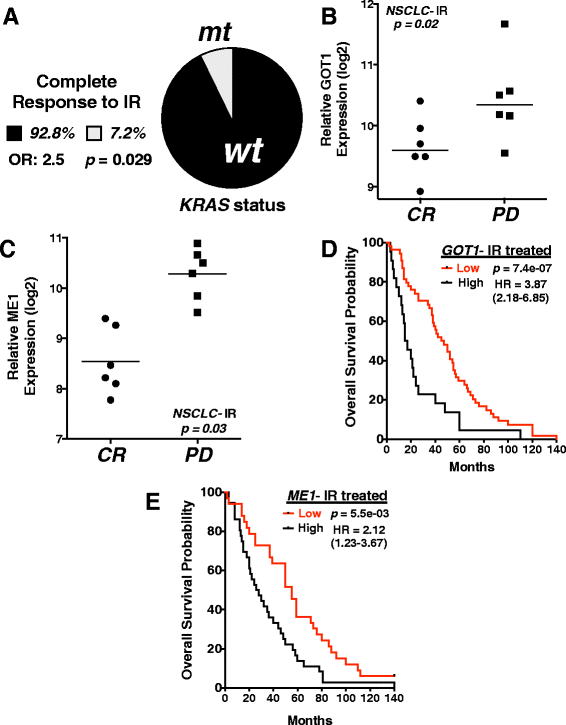
Findings: A doxycycline-inducible mouse model of KRAS (G12D) driven NSCLC and patient data was analyzed from multiple publicly accessible databases including TCGA, CCLE, NCBI GEO and Project Achilles. ME1 expression was found to be mutant KRAS associated in both a NSCLC mouse model and human NSCLC cancer cell lines. Perturbing glutamine metabolism sensitized mutant KRAS, but not wild-type KRAS NSCLC cell lines to radiation treatment. NSCLC survival analysis revealed that patients with elevated ME1 and GOT1 expression had significantly worse outcomes after radiotherapy, but this was not seen after chemotherapy alone.
Conclusions: KRAS driven glutamine metabolism genes, specifically ME1 and GOT1 reactions, may be a predictive marker and potential therapeutic target for radiotherapy in NSCLC.
Figures
Similar articles
-
KRAS driven expression signature has prognostic power superior to mutation status in non-small cell lung cancer.Int J Cancer. 2017 Feb 15;140(4):930-937. doi: 10.1002/ijc.30509. Epub 2016 Nov 23. Int J Cancer. 2017. PMID: 27859136 Free PMC article.
-
Plasma miRNAs in predicting radiosensitivity in non-small cell lung cancer.Tumour Biol. 2016 Sep;37(9):11927-11936. doi: 10.1007/s13277-016-5052-8. Epub 2016 Apr 13. Tumour Biol. 2016. PMID: 27075472 Free PMC article.
-
Up-regulation of SRPK1 in non-small cell lung cancer promotes the growth and migration of cancer cells.Tumour Biol. 2016 Jun;37(6):7287-93. doi: 10.1007/s13277-015-4510-z. Epub 2015 Dec 14. Tumour Biol. 2016. PMID: 26666824
-
[BRCA1: a new predictive genomic marker for chemotherapy and radiotherapy of non-small cell lung cancer].Zhongguo Fei Ai Za Zhi. 2012 Aug;15(8):481-90. doi: 10.3779/j.issn.1009-3419.2012.08.06. Zhongguo Fei Ai Za Zhi. 2012. PMID: 22901997 Free PMC article. Review. Chinese.
-
Predictive testing of radiosensitivity in non-small cell carcinoma of the lung.Lung Cancer. 1994 Mar;10 Suppl 1:S83-90. doi: 10.1016/0169-5002(94)91670-5. Lung Cancer. 1994. PMID: 8087531 Review.
Cited by
-
Identification and Prognostic Value Exploration of Radiotherapy Sensitivity-Associated Genes in Non-Small-Cell Lung Cancer.Biomed Res Int. 2021 Sep 2;2021:5963868. doi: 10.1155/2021/5963868. eCollection 2021. Biomed Res Int. 2021. PMID: 34518802 Free PMC article.
-
NADPH homeostasis in cancer: functions, mechanisms and therapeutic implications.Signal Transduct Target Ther. 2020 Oct 7;5(1):231. doi: 10.1038/s41392-020-00326-0. Signal Transduct Target Ther. 2020. PMID: 33028807 Free PMC article. Review.
-
Simultaneous perturbation of the MAPK and the PI3K/mTOR pathways does not lead to increased radiosensitization.Radiat Oncol. 2015 Oct 24;10:214. doi: 10.1186/s13014-015-0514-5. Radiat Oncol. 2015. PMID: 26498922 Free PMC article.
-
MicroRNA-30a attenuates mutant KRAS-driven colorectal tumorigenesis via direct suppression of ME1.Cell Death Differ. 2017 Jul;24(7):1253-1262. doi: 10.1038/cdd.2017.63. Epub 2017 May 5. Cell Death Differ. 2017. PMID: 28475173 Free PMC article.
-
Extracellular citrate and metabolic adaptations of cancer cells.Cancer Metastasis Rev. 2021 Dec;40(4):1073-1091. doi: 10.1007/s10555-021-10007-1. Epub 2021 Dec 21. Cancer Metastasis Rev. 2021. PMID: 34932167 Free PMC article. Review.
References
-
- Rowell NP, Williams CJ. Radical radiotherapy for stage I/II non-small cell lung cancer in patients not sufficiently fit for or declining surgery (medically inoperable) Cochrane Database Syst Rev. 2001;1:CD002935. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

