A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis
- PMID: 26173827
- PMCID: PMC4532527
- DOI: 10.1113/jphysiol.2014.278317
A mathematical model of salt-sensitive hypertension: the neurogenic hypothesis
Abstract
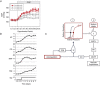
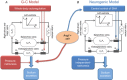
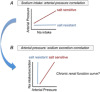
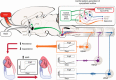
Salt sensitivity of arterial pressure (salt-sensitive hypertension) is a serious global health issue. The causes of salt-sensitive hypertension are extremely complex and mathematical models can elucidate potential mechanisms that are experimentally inaccessible. Until recently, the only mathematical model for long-term control of arterial pressure was the model of Guyton and Coleman; referred to as the G-C model. The core of this model is the assumption that sodium excretion is driven by renal perfusion pressure, the so-called 'renal function curve'. Thus, the G-C model dictates that all forms of hypertension are due to a primary shift of the renal function curve to a higher operating pressure. However, several recent experimental studies in a model of hypertension produced by the combination of a high salt intake and administration of angiotensin II, the AngII-salt model, are inconsistent with the G-C model. We developed a new mathematical model that does not limit the cause of salt-sensitive hypertension solely to primary renal dysfunction. The model is the first known mathematical counterexample to the assumption that all salt-sensitive forms of hypertension require a primary shift of renal function: we show that in at least one salt-sensitive form of hypertension the requirement is not necessary. We will refer to this computational model as the 'neurogenic model'. In this Symposium Review we discuss how, despite fundamental differences between the G-C model and the neurogenic model regarding mechanisms regulating sodium excretion and vascular resistance, they generate similar haemodynamic profiles of AngII-salt hypertension. In addition, the steady-state relationships between arterial pressure and sodium excretion, a correlation that is often erroneously presented as the 'renal function curve', are also similar in both models. Our findings suggest that salt-sensitive hypertension is not due solely to renal dysfunction, as predicted by the G-C model, but may also result from neurogenic dysfunction.
© 2014 The Authors. The Journal of Physiology © 2014 The Physiological Society.
Figures

References
-
- Barrett CJ. Malpas SC. Problems, possibilities, and pitfalls in studying the arterial baroreflexes’ influence over long-term control of blood pressure. Am J Physiol Regul Integr Comp Physiol. 2005;288:R837–R845. - PubMed
-
- Bie P. Blood volume, blood pressure and total body sodium: internal signalling and output control. Exp Physiol. 2009;195:187–196. - PubMed
-
- DeClue JW, Guyton AC, Cowley AW, Jr, Coleman TG, Norman RA., Jr McCaa RE. Subpressor angiotensin infusion, renal sodium handling, and salt-induced hypertension in the dog. Circ Res. 1978;43:503–512. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

