Identification of tool use acquisition-associated genes in the primate neocortex
- PMID: 26173833
- PMCID: PMC11520950
- DOI: 10.1111/dgd.12227
Identification of tool use acquisition-associated genes in the primate neocortex
Abstract
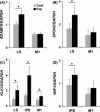
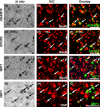
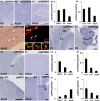
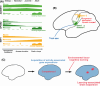
Japanese macaques are able to learn how to use rakes to take food after only a few weeks of training. Since tool-use training induced rapid morphological changes in some restricted brain areas, this system will be a good model for studying the neural basis of plasticity in human brains. To examine the mechanisms of tool-use associated brain expansion on the molecular and cellular level, here, we performed comprehensive analysis of gene expressions with microarray. We identified various transcripts showing differential expression between trained and untrained monkeys in the region around the lateral and intraparietal sulci. Among candidates, we focused on genes related to synapse formation and function. Using quantitative reverse transcription-polymerase chain reaction and histochemical analysis, we confirmed at least three genes (ADAM19, SPON2, and WIF1) with statistically different expression levels in neurons and glial cells. Comparative analysis revealed that tool use-associated genes were more obviously expressed in macaque monkeys than marmosets or mice. Thus, our findings suggest that cognitive tasks induce structural changes in the neocortex via gene expression, and that learning-associated genes innately differ with relation to learning ability.
Keywords: gene expression; macaque; microarray; parietal cortex; tool use.
© 2015 The Authors Development, Growth & Differentiation published by Wiley Publishing Asia Pty Ltd on behalf of Japanese Society of Developmental Biologists.
Figures


Similar articles
-
An evolutionarily conserved sexual signature in the primate brain.PLoS Genet. 2008 Jun 20;4(6):e1000100. doi: 10.1371/journal.pgen.1000100. PLoS Genet. 2008. PMID: 18566661 Free PMC article.
-
Differentially expressed genes among motor and prefrontal areas of macaque neocortex.Biochem Biophys Res Commun. 2007 Oct 26;362(3):665-9. doi: 10.1016/j.bbrc.2007.08.039. Epub 2007 Aug 20. Biochem Biophys Res Commun. 2007. PMID: 17761142
-
Gray and white matter changes associated with tool-use learning in macaque monkeys.Proc Natl Acad Sci U S A. 2009 Oct 27;106(43):18379-84. doi: 10.1073/pnas.0909751106. Epub 2009 Oct 9. Proc Natl Acad Sci U S A. 2009. PMID: 19820167 Free PMC article.
-
The neuroscience of primate intellectual evolution: natural selection and passive and intentional niche construction.Philos Trans R Soc Lond B Biol Sci. 2008 Jun 27;363(1500):2229-41. doi: 10.1098/rstb.2008.2274. Philos Trans R Soc Lond B Biol Sci. 2008. PMID: 18426757 Free PMC article. Review.
-
Molecular analysis of developmental plasticity in neocortex.J Neurobiol. 1999 Oct;41(1):135-47. J Neurobiol. 1999. PMID: 10504201 Free PMC article. Review.
Cited by
-
Simulation and social network analysis provide insight into the acquisition of tool behaviour in hybrid macaques.Proc Biol Sci. 2023 Mar 29;290(1995):20222276. doi: 10.1098/rspb.2022.2276. Epub 2023 Mar 29. Proc Biol Sci. 2023. PMID: 36987645 Free PMC article.
-
Derivation of induced pluripotent stem cells in Japanese macaque (Macaca fuscata).Sci Rep. 2018 Aug 15;8(1):12187. doi: 10.1038/s41598-018-30734-w. Sci Rep. 2018. PMID: 30111816 Free PMC article.
-
The clinical significance of spondin 2 eccentric expression in peripheral blood mononuclear cells in bronchial asthma.J Clin Lab Anal. 2021 Jun;35(6):e23764. doi: 10.1002/jcla.23764. Epub 2021 May 16. J Clin Lab Anal. 2021. PMID: 33998076 Free PMC article.
-
Secondary somatosensory cortex of primates: beyond body maps, toward conscious self-in-the-world maps.Exp Brain Res. 2020 Feb;238(2):259-272. doi: 10.1007/s00221-020-05727-9. Epub 2020 Jan 21. Exp Brain Res. 2020. PMID: 31960104 Free PMC article. Review.
References
-
- Binkofski, F. , Buccino, G. , Posse, S. , Seitz, R. J. , Rizzolatti, G. & Freund, H. 1999a. A fronto‐parietal circuit for object manipulation in man: evidence from an fMRI‐study. Eur. J. Neurosci. 11, 3276–3286. - PubMed
-
- Binkofski, F. , Buccino, G. , Stephan, K. M. , Rizzolatti, G. , Seitz, R. J. & Freund, H. J. 1999b. A parieto‐premotor network for object manipulation: evidence from neuroimaging. Exp. Brain Res. 128, 210–213. - PubMed
-
- Borra, E. , Belmalih, A. , Calzavara, R. , Gerbella, M. , Murata, A. , Rozzi, S. & Luppino, G. 2008. Cortical connections of the macaque anterior intraparietal (AIP) area. Cereb. Cortex 18, 1094–1111. - PubMed
-
- Burton, H. , Abend, N. S. , MacLeod, A. M. , Sinclair, R. J. , Snyder, A. Z. & Raichle, M. E. 1999. Tactile attention tasks enhance activation in somatosensory regions of parietal ortex: a positron emission tomography study. Cereb. Cortex 9, 662–674. - PubMed
Publication types
MeSH terms
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

