Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients With Non-Small Cell Lung Cancer
- PMID: 26173839
- PMCID: PMC4524769
- DOI: 10.1634/theoncologist.2015-0058
Nutritional Status, Body Surface, and Low Lean Body Mass/Body Mass Index Are Related to Dose Reduction and Severe Gastrointestinal Toxicity Induced by Afatinib in Patients With Non-Small Cell Lung Cancer
Abstract
Background: The main reason for dose reduction of afatinib is gastrointestinal toxicity (GT). In a phase II study, we analyzed anthropometrical, nutritional, and biochemical factors associated with GT induced by afatinib.
Materials and methods: Patients diagnosed with non-small cell lung cancer who progressed to prior chemotherapy received 40 mg of afatinib. Malnutrition was determined by Subjective Global Assessment, and lean body mass (LBM) was determined by computed tomography scan analysis using a pre-established Hounsfield unit threshold. Toxicity was obtained during four cycles by Common Terminology Criteria for Adverse Events.
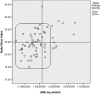
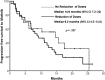
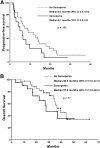
Results: Eighty-four patients were enrolled. Afatinib was administered as the second, third, and fourth line of treatment in 54.8%, 38.1%, and 7.12% of patients, respectively. Severe diarrhea, mucositis, and overall severe GT were present in 38.9%, 28.8%, and 57.5%, respectively. Of the patients, 50% developed dose-limiting toxicity (DLT). Patients with malnutrition have higher risk for severe GT. Patients with lower LBM and body mass index developed more DLT (71.4% vs. 18.8%).
Conclusion: Malnutrition is associated with a higher risk of severe GT induced by afatinib. Determination of nutritional status and body composition are helpful in identifying patients at higher risk of severe GT and could allow initiating treatment with lower doses according to tolerance.
Keywords: Afatinib; Gastrointestinal toxicity; Malnourishment; Non-small cell lung cancer; Nutritional status; Phase II trial.
©AlphaMed Press.
Conflict of interest statement
Disclosures of potential conflicts of interest may be found at the end of this article.
Figures
Comment in
-
Sarcopenia Is a Condition With Increasing Importance in Medical Oncology.Oncologist. 2016 Feb;21(2):e1. doi: 10.1634/theoncologist.2015-0431. Epub 2016 Jan 14. Oncologist. 2016. PMID: 26768481 Free PMC article.
-
In Reply.Oncologist. 2016 Feb;21(2):e2. doi: 10.1634/theoncologist.2015-0465. Epub 2016 Jan 14. Oncologist. 2016. PMID: 26768484 Free PMC article.
References
-
- Siegel R, Ma J, Zou Z, et al. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. - PubMed
-
- Arrieta O, Guzmán-de Alba E, Alba-López LF, et al. National consensus of diagnosis and treatment of non-small cell lung cancer [in Spanish] Rev Invest Clin. 2013;65(suppl 1):S5–S84. - PubMed
-
- Arrieta Ó, Núñez-Valencia C, Reynoso-Erazo L, et al. Health-related quality of life in patients with lung cancer: Validation of the Mexican-Spanish version and association with prognosis of the EORTC QLQ-LC13 questionnaire. Lung Cancer. 2012;77:205–211. - PubMed
-
- Schiller JH, Harrington D, Belani CP, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346:92–98. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

