A comparative study of the localization and membrane topology of members of the RIFIN, STEVOR and PfMC-2TM protein families in Plasmodium falciparum-infected erythrocytes
- PMID: 26173856
- PMCID: PMC4502930
- DOI: 10.1186/s12936-015-0784-2
A comparative study of the localization and membrane topology of members of the RIFIN, STEVOR and PfMC-2TM protein families in Plasmodium falciparum-infected erythrocytes
Abstract
Background: Variant surface antigens (VSA) exposed on the membrane of Plasmodium falciparum infected erythrocytes mediate immune evasion and are important pathogenicity factors in malaria disease. In addition to the well-studied PfEMP1, the small VSA families RIFIN, STEVOR and PfMC-2TM are assumed to play a role in this process.
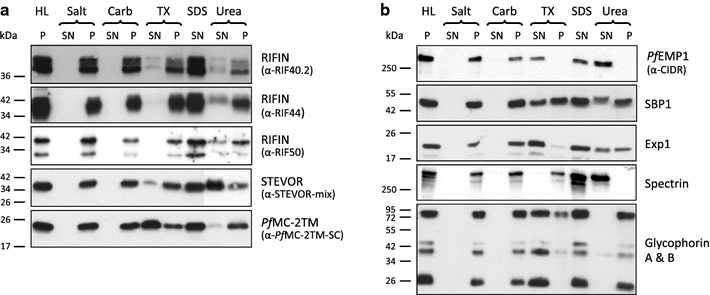
Methods: This study presents a detailed comparative characterization of the localization, membrane topology and extraction profile across the life cycle of various members of these protein families employing confocal microscopy, immunoelectron microscopy and immunoblots.
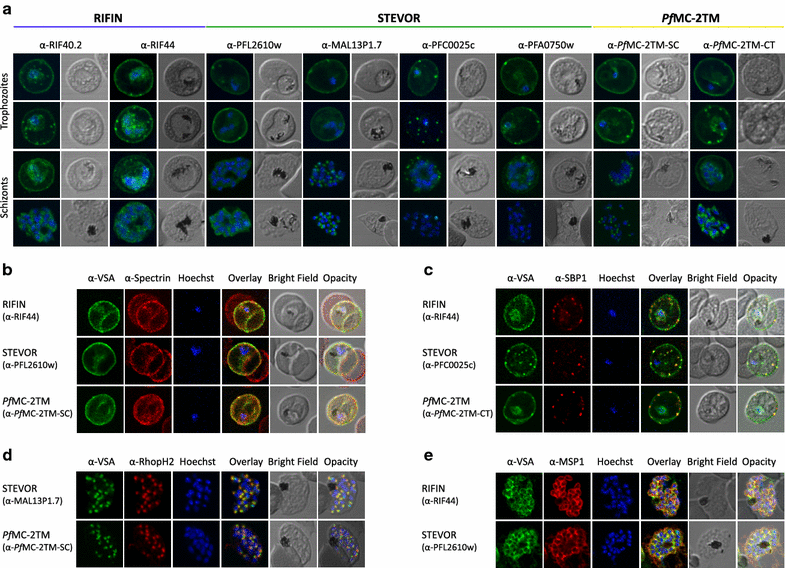
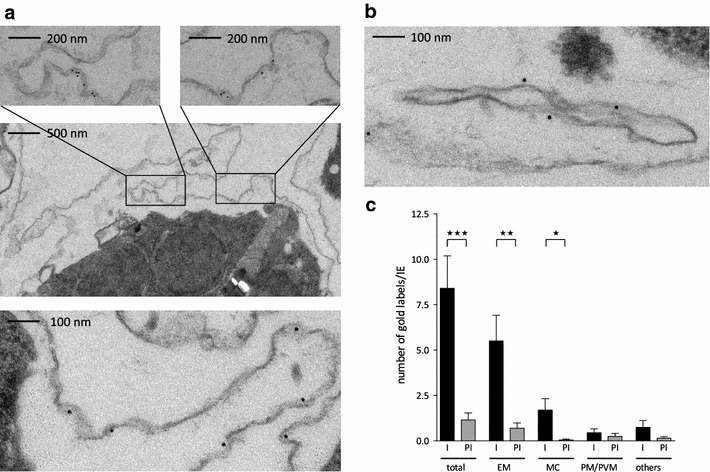
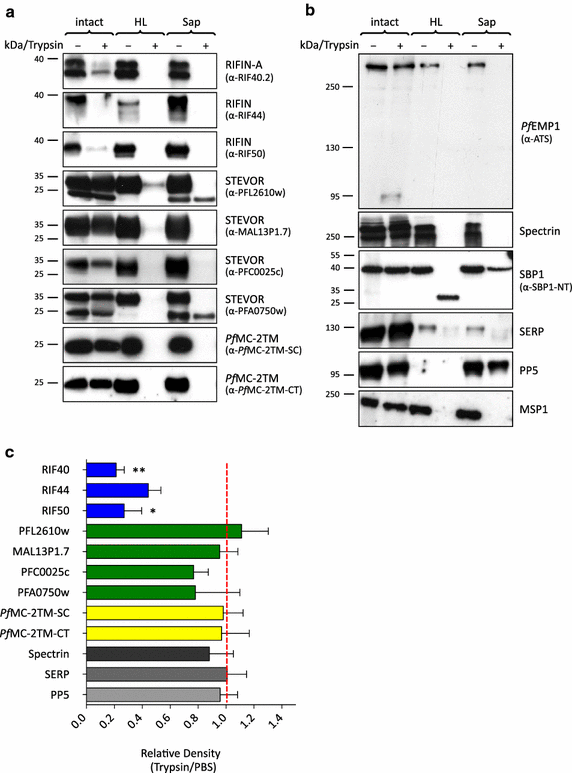
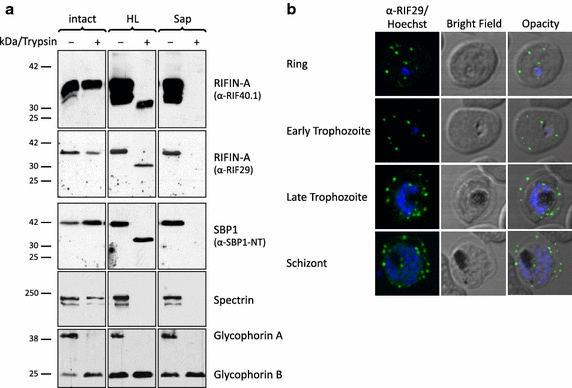
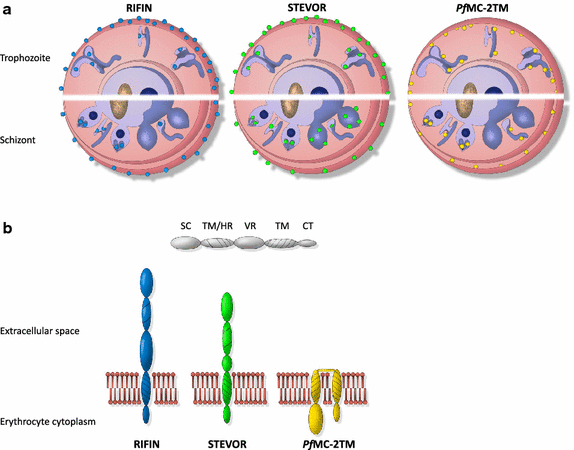
Results: The presented data reveal a clear association of variants of the RIFIN, STEVOR and PfMC-2TM proteins with the host cell membrane and topological studies indicate that the semi-conserved N-terminal region of RIFINs and some STEVOR proteins is exposed at the erythrocyte surface. At the Maurer's clefts, the semi-conserved N-terminal region as well as the variable stretch of RIFINs appears to point to the lumen away from the erythrocyte cytoplasm. These results challenge the previously proposed two transmembrane topology model for the RIFIN and STEVOR protein families and suggest that only one hydrophobic region spans the membrane. In contrast, PfMC-2TM proteins indeed seem to be anchored by two hydrophobic stretches in the host cell membrane exposing just a few, variable amino acids at the surface of the host cell.
Conclusion: Together, the host cell surface exposure and topology of RIFIN and STEVOR proteins suggests members of these protein families may indeed be involved in immune evasion of the infected erythrocyte, whereas members of the PfMC-2TM family seem to bear different functions in parasite biology.
Figures
References
-
- Bruce-Chwatt LJ. A longitudinal survey of natural malaria infection in a group of West African adults. West Afr Med J. 1963;12:199–217. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

