The pharmacogenetic control of antiplatelet response: candidate genes and CYP2C19
- PMID: 26173871
- PMCID: PMC4829114
- DOI: 10.1517/17425255.2015.1068757
The pharmacogenetic control of antiplatelet response: candidate genes and CYP2C19
Abstract
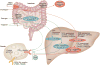
Introduction: Aspirin, clopidogrel, prasugrel and ticagrelor are antiplatelet agents for the prevention of ischemic events in patients with acute coronary syndromes (ACS), percutaneous coronary intervention (PCI) and other indications. Variability in response is observed to different degrees with these agents, which can translate to increased risks for adverse cardiovascular events. As such, potential pharmacogenetic determinants of antiplatelet pharmacokinetics, pharmacodynamics and clinical outcomes have been actively studied.
Areas covered: This article provides an overview of the available antiplatelet pharmacogenetics literature. Evidence supporting the significance of candidate genes and their potential influence on antiplatelet response and clinical outcomes are summarized and evaluated. Additional focus is directed at CYP2C19 and clopidogrel response, including the availability of clinical testing and genotype-directed antiplatelet therapy.
Expert opinion: The reported aspirin response candidate genes have not been adequately replicated and few candidate genes have thus far been implicated in prasugrel or ticagrelor response. However, abundant data support the clinical validity of CYP2C19 and clopidogrel response variability among ACS/PCI patients. Although limited prospective trial data are available to support the utility of routine CYP2C19 testing, the increased risks for reduced clopidogrel efficacy among ACS/PCI patients that carry CYP2C19 loss-of-function alleles should be considered when genotype results are available.
Keywords: CYP2C19; antiplatelet agents; aspirin; candidate genes; clopidogrel; pharmacogenetics; pharmacogenomics; prasugrel; ticagrelor.
Conflict of interest statement
JP Lewis receives support from NIH for antiplatelet pharmacogenomics research. J-S Hulot has received research grant support from Biotronik, and consulting fees from Daiichi Sankyo. SA Scott receives support from NIH for antiplatelet pharmacogenomics research and is an Associate Director of a clinical laboratory that performs
Figures

Similar articles
-
Personalizing antiplatelet prescribing using genetics for patients undergoing percutaneous coronary intervention.Expert Rev Cardiovasc Ther. 2017 Aug;15(8):581-589. doi: 10.1080/14779072.2017.1355236. Epub 2017 Jul 19. Expert Rev Cardiovasc Ther. 2017. PMID: 28699807 Free PMC article. Review.
-
Multisite Investigation of Outcomes With Implementation of CYP2C19 Genotype-Guided Antiplatelet Therapy After Percutaneous Coronary Intervention.JACC Cardiovasc Interv. 2018 Jan 22;11(2):181-191. doi: 10.1016/j.jcin.2017.07.022. Epub 2017 Nov 1. JACC Cardiovasc Interv. 2018. PMID: 29102571 Free PMC article. Clinical Trial.
-
Economic Evaluations of CYP2C19 Genotype-Guided Antiplatelet Therapy Compared to the Universal Use of Antiplatelets in Patients With Acute Coronary Syndrome: A Systematic Review.J Cardiovasc Pharmacol Ther. 2020 May;25(3):201-211. doi: 10.1177/1074248420902298. Epub 2020 Feb 6. J Cardiovasc Pharmacol Ther. 2020. PMID: 32027168
-
Impact of CYP2C19 genetic testing on provider prescribing patterns for antiplatelet therapy after acute coronary syndromes and percutaneous coronary intervention.Circ Cardiovasc Qual Outcomes. 2013 Nov;6(6):694-9. doi: 10.1161/CIRCOUTCOMES.113.000321. Epub 2013 Nov 5. Circ Cardiovasc Qual Outcomes. 2013. PMID: 24192573
-
CYP2C19 polymorphisms and coronary heart disease risk factors synergistically impact clopidogrel response variety after percutaneous coronary intervention.Coron Artery Dis. 2014 Aug;25(5):412-20. doi: 10.1097/MCA.0000000000000092. Coron Artery Dis. 2014. PMID: 24608794
Cited by
-
Association of FMO3 rs1736557 polymorphism with clopidogrel response in Chinese patients with coronary artery disease.Eur J Clin Pharmacol. 2021 Mar;77(3):359-368. doi: 10.1007/s00228-020-03024-6. Epub 2020 Oct 21. Eur J Clin Pharmacol. 2021. PMID: 33089397
-
MicroRNA as Potential Biomarkers of Platelet Function on Antiplatelet Therapy: A Review.Front Physiol. 2021 Apr 15;12:652579. doi: 10.3389/fphys.2021.652579. eCollection 2021. Front Physiol. 2021. PMID: 33935804 Free PMC article. Review.
-
Exome sequencing of extreme clopidogrel response phenotypes identifies B4GALT2 as a determinant of on-treatment platelet reactivity.Clin Pharmacol Ther. 2016 Sep;100(3):287-94. doi: 10.1002/cpt.401. Epub 2016 Jun 20. Clin Pharmacol Ther. 2016. PMID: 27213804 Free PMC article.
-
Race and Drug Toxicity: A Study of Three Cardiovascular Drugs with Strong Pharmacogenetic Recommendations.J Pers Med. 2021 Nov 18;11(11):1226. doi: 10.3390/jpm11111226. J Pers Med. 2021. PMID: 34834577 Free PMC article.
-
Comparison of a rapid point-of-care and two laboratory-based CYP2C19*2 genotyping assays for personalisation of antiplatelet therapy.Int J Clin Pharm. 2016 Apr;38(2):414-20. doi: 10.1007/s11096-016-0269-6. Epub 2016 Mar 15. Int J Clin Pharm. 2016. PMID: 26980150
References
-
- Jennings LK. Mechanisms of platelet activation: need for new strategies to protect against platelet-mediated atherothrombosis. Thrombosis and haemostasis. 2009 Aug;102(2):248–57. - PubMed
-
- Buonamici P, Marcucci R, Migliorini A, et al. Impact of platelet reactivity after clopidogrel administration on drug-eluting stent thrombosis. J Am Coll Cardiol. 2007 Jun 19;49(24):2312–7. - PubMed
-
- Tantry US, Bonello L, Aradi D, et al. Consensus and update on the definition of on-treatment platelet reactivity to adenosine diphosphate associated with ischemia and bleeding. J Am Coll Cardiol. 2013 Dec 17;62(24):2261–73. - PubMed
-
- Tantry US, Mahla E, Gurbel PA. Aspirin resistance. Prog Cardiovasc Dis. 2009 Sep-Oct;52(2):141–52. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous
